La amiloidosis secundaria o AA es una grave complicación de la enfermedad de Crohn (EC) evolucionada para la que no se dispone de un tratamiento eficaz. Presentamos un caso excepcional de un varón de 33 años afecto de insuficiencia renal moderada y proteinuria, que fue diagnosticado simultáneamente de nefropatía amiloide AA y de EC oligosintomática. Fue tratado con infliximab, 5 mg/kg/8 semanas durante 4 años, azatioprina 1-1,5 mg/kg/día (primer año) y un bloqueante del sistema renina-angiotensina-aldosterona, sin complicaciones. El tratamiento se siguió de una reducción de la proteinuria, mejoría de la función renal y de los parámetros inflamatorios a lo largo del tiempo. A propósito de este caso revisamos la literatura médica y concluimos que infliximab puede ser de utilidad para el tratamiento precoz de la amiloidosis secundaria en la EC.
Secondary amyloidosis (AA) is a severe complication of progressed Crohn’s disease (CD) for which no effective treatment exists. We present the exceptional case of a 33 year-old male with moderate renal failure and proteinuria, who was simultaneously diagnosed with AA amyloid nephropathy and oligosymptomatic CD. He was treated with infliximab at 5mg/kg/8 weeks for 4 years, azathioprine at 1-1.5mg/kg/day (first year) and renin-angiotensin-aldosterone system blockers, with no complications. Treatment caused a decrease in proteinuria, improved renal function, and improved inflammatory parameters over time. Inspired by this case, we performed a review of the medical literature and found that infliximab could be a useful tool in the early treatment of amyloidosis secondary to CD.
INTRODUCTION
Secondary amyloidosis or AA is a rare but severe complication associated with Crohn’s disease (CD). The deposition of amyloid material in AA amyloidosis mainly affects the kidneys and the major clinical manifestations are proteinuria and renal failure. The progression of chronic kidney disease associated with amyloidosis secondary to CD in the absence of an effective treatment leads to high morbidity and mortality rates.1-3
However, anti-tumour necrosis factor-alpha (anti-TNFα) agents, infliximab in particular, have been successfully used in recent years to treat AA amyloidosis in some inflammatory rheumatic diseases4 and also in some cases of CD.5-8
We present the case of a patient simultaneously diagnosed with oligosymptomatic CD and AA renal amyloidosis, successfully treated with infliximab over 4 years with a good response and absence of complications.
CASE REPORT
A 33-year-old male was referred to the nephrology unit in late 2006 due to elevated serum creatinine (SCr) levels, 1.4mg/dl. He had no history or clinical manifestations of urological or kidney disease. Five years previously, his SCr had been measured at 0.9mg/dl.
At 18-years-old, he was diagnosed with iron-deficiency anaemia and gastro-oesophageal reflux. In the past 4 years he reported suffering from dysphagia and postprandial heaviness, without any other gastrointestinal manifestations; and mechanical back pain. He had no febrile episodes, joint inflammation, skin lesions, serositis, or problems with any other body organs or systems.
He had a family history of ankylosing spondylitis in several males on his father’s side; a paternal uncle was also diagnosed with stage 5 chronic kidney disease secondary to nephropathy caused by analgesic use.
Normal physical examination, blood pressure: 130/79mm Hg, body mass index: 21kg/m2.
Diagnostic tests
Blood test: SCr: 1.47mg/dl; MDRD-4 estimated glomerular filtration rate (eFGR): 59ml/min/1.73m2; urea: 33mg/dl; ions, liver enzymes and lipids: normal. Total proteins, proteinogram, immunoglobulins and complement: normal. Negative rheumatoid factor. C-reactive protein (CRP): 13mg/l (normal value [NV]<10mg/l). Antinuclear antibodies, anti-DNA antibodies, anti-neutrophil cytoplasmic antibodies and HLA-B27 histocompatibility antigens were negative. Iron parameters: serum iron: 25µg/dl (NV: 40-60); ferritin: 38ng/ml (NV: 20-300), transferrin saturation index: 12%. Vitamin B12: 181pg/ml (NV: 208-930) and folic acid: 3.3ng/ml (NV: 7.2-15).
Urine test: proteinuria: 200mg/24h; albuminuria: 47mg/24h, with no monoclonal free light chains. Normal urine sediment.
Chest and spine x-rays showed no significant alterations. Ultrasound scans of the abdomen, kidneys and urinary passages: normal. Analysis by the rheumatology department ruled out ankylosing spondylitis.
Repeat tests after two months showed: SCr: 1.64mg/dl; eFGR (MDRD-4): 52ml/min/1.73m2; proteinuria: 316mg/24h; albuminuria: 163mg/24h. And 4 months later: SCr: 1.77mg/dl; eFGR (MDRD-4): 47ml/min/1.73m2; proteinuria: 640mg/24h. The iron, vitamin B12 and folic acid deficiencies were partially corrected with oral supplements.
In light of the persistent gastrointestinal symptoms (dysphagia, dyspepsia) and suspected intestinal malabsorption (iron and vitamin deficiency), we decided to perform an endoscopy of the digestive tract and take biopsies. The main alterations found in the mucosa of the terminal ileum were compatible with CD: ulcerated villi, lymphoplasmacytic inflammation in the lamina propria, neutrophil infiltration (crypt abscesses) and epithelioid histiocyte granulomas. A deposit of material with staining characteristics (Congo red and immunohistochemical) of AA amyloidosis, and no signs of inflammation, was found in the rectal mucosa, in the blood vessel walls of the lamina propria.
A percutaneous renal biopsy was also performed in light of the unfavourable progression of the renal parameters (renal function and proteinuria), which showed: 7 glomeruli, 3 with virtually global glomerulosclerosis and the remaining 4 with small eosinophilic deposits in the hilum of the glomerulus; patchy areas of interstitial fibrosis; and eosinophilic deposits in the walls of the blood vessels that were larger and more intense than the glomerular ones. The deposits tested positive for Congo red with green birefringence under polarised light and immunohistochemical staining detected the presence of amyloid A protein.
In the subsequent evolution of this case, the patient had no symptoms or clinical signs suggestive of any other type of inflammatory, infectious or tumour disease, or familial Mediterranean fever.
With the diagnosis of CD and secondary AA amyloidosis, it was decided to pursue aetiological treatment of the CD based on intravenous infliximab at 5mg/kg every 2 months, azathioprine at 1-1.5mg/kg/day (for the first year only, suspended due to leukopenia) and renin-angiotensin-aldosterone system blockers. The renal parameters improved (Figure 1), as did the inflammatory markers (CRP: 5mg/l, and serum amyloid A protein <5mg/l), and are maintained after 4 years of monitoring. There have been no major complications of the CD or side effects from the medication.
DISCUSSION
Between 0.9% and 3% of patients with CD develop secondary amyloidosis,1-3 a rate that is 2.5-3 times higher in men.1,2
The average time from the diagnosis of CD to the appearance of amyloidosis is 10-15 years1,2; in exceptional cases, the diagnosis of both clinical entities occurs simultaneously2 and it has even been suggested that amyloidosis can precede CD in rare cases.9 In our patient, both entities were diagnosed at the same time, although minor and non-specific digestive manifestations had been present for several years. In the two longest studies published to date,1,2 two thirds of people with amyloidosis secondary to CD had previous suppurative complications (fistulas or abscesses), and half or more had extraintestinal manifestations; from which we can deduce that AA amyloidosis in CD can also develop in the absence of chronic infections or extraintestinal complications, as in our case. In line with the foregoing, it would appear that AA amyloid nephropathy can first appear as an early complication in CD and not necessarily be associated with a clinically manifest, extensive and aggressive disease.
The main objectives of secondary renal amyloidosis treatment are to induce and maintain the remission of the primary disease, prevent renal deposition of amyloid A proteins, and stop or reverse the progression of chronic kidney disease. The effect of intestinal resection to prevent or treat renal amyloidosis in DC is controversial,1,3 and the majority of authors advocate drug treatment as the treatment of choice. Drugs like colchicine, azathioprine and dimethyl sulfoxide can slow down the progression of amyloid nephropathy, but their effectiveness has not been fully demonstrated.3 Eprodisate, the first drug in a new class of compounds, inhibits amyloid protein polymerisation and deposition in tissues and slows down the decline in the glomerular filtration rate in AA amyloidosis patients, but it has little effect on proteinuria and survival.10
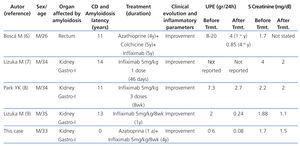
In recent years, the anti-TNFα agent infliximab has been used successfully to treat isolated cases of renal amyloidosis secondary to CD. We conducted a PubMed search with the words “systemic or secondary amyloidosis, Crohn’s disease, infliximab” and selected those cases published in English not associated with other rheumatic inflammatory or infectious diseases or with familial Mediterranean fever.5-8 The main clinical characteristics of the four cases found in addition to our own are summarised in Table 1. In all these cases, treatment with infliximab was started soon after the diagnosis with amyloidosis and was followed by rapid and maintained clinical improvement of the intestinal disease and the laboratory inflammatory parameters, a rapid decrease in proteinuria and improved renal function with no complications associated with the medication. One of these studies showed a decrease in the amyloid deposits in the intestinal mucosa after 5 years of treatment with infliximab.5
The most common adverse effects relating to ongoing infliximab use in patients with amyloidosis and rheumatic diseases are an increased susceptibility to infections and the drug’s loss of effectiveness.4 In cases of resistance or loss of effectiveness or adverse effects in patients with CD, another anti-TNFα, adalimumab, can be used as an alternative.11,12
It has been hypothesised that anti-TNFα agents can improve amyloid nephropathy in inflammatory diseases through two mechanisms: 1) by reducing the glomerular inflammation and the increase in the glomerular permeability to albumin induced by TNFα cytokines and interleukin-6; and 2) by reducing the synthesis of acute-phase proteins mediated by the same cytokines.4 Indeed, it has been demonstrated that the serum amyloid A protein level reached is a prognostic factor in AA amyloidosis; mean levels below 10mg/l are associated with a higher likelihood of regression of the amyloid deposits and higher survival rates.13
To conclude, AA renal amyloidosis can first appear as an early complication in patients with CD, even in those patients with a disease that presents mild clinical symptoms. It would appear appropriate to recommend renal function and urinary protein excretion tests for patients diagnosed with CD at the time of diagnosis and at regular intervals thereafter with a view to diagnosing and, where applicable, starting early treatment on any associated kidney disease. Infliximab could be a useful and effective tool in the early treatment of amyloidosis secondary to CD and provides encouraging prospects.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Evolution of the renal parameters of 5 patients with AA amyloidosis and Crohns disease treated with infliximab
Figure 1. Evolution of serum creatinine and urinary protein excretion before and after treatment with infliximab