El interés principal en el desarrollo de nuevos inmunosupresores para el trasplante renal no sólo radica en la mejora de los resultados a corto plazo, sino también en un mejor perfil de seguridad, una menor nefrotoxicidad y un mejor perfil cardiovascular y metabólico. Belatacept es una proteína de fusión que bloquea la coestimulación de los linfocitos al unirse a los antígenos CD80 y CD86. En los estudios clínicos, especialmente BENEFIT y BENEFIT-EXE, se ha demostrado que belatacept preserva la función y la estructura del injerto renal y que sus efectos se mantienen a largo plazo. En comparación con los inhibidores de la calcineurina, belatacept se asocia a una menor incidencia de nefropatía crónica del injerto y ofrece un perfil cardiovascular y metabólico más favorable. Su eficacia y seguridad se mantienen en trasplantes renales de donantes con criterios ampliados.
The development of new immunosuppressants for renal transplantation is aimed not only at improving short-term outcomes, but also at achieving better safety, cardiovascular, and metabolic profiles and at decreasing nephrotoxicity. Belatacept is a fusion protein that inhibits T cell activation by binding to CD80 and CD86 antigens. Clinical trials, particularly the BENEFIT and BENEFIT-EXT studies, have shown that belatacept preserves function and structure in renal grafts. The effects of belatacept provide long-term, sustained results, and the safety and efficacy of this drug have been demonstrated in cases of renal transplantation from expanded criteria donors. Compared to calcineurin inhibitors, belatacept is associated with a lower incidence of chronic allograft nephropathy and a more favourable cardiovascular and metabolic profile.
INTRODUCTION
The objective of immunosuppression in kidney transplantation is to prevent and treat acute rejection and avoid chronic graft damage, thus minimising the adverse effects of immunosuppressants. The introduction of cyclosporine, tacrolimus, and mycophenolate mofetil (MMF) reduced rates of acute rejection and improved short and mid-term graft survival,1,2 as reported by data from the Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) in the United States,3 and the Grupo Español para el Estudio de la Nefropatía Crónica del Trasplante (Spanish Chronic Allograft Nephropathy Study Group).4
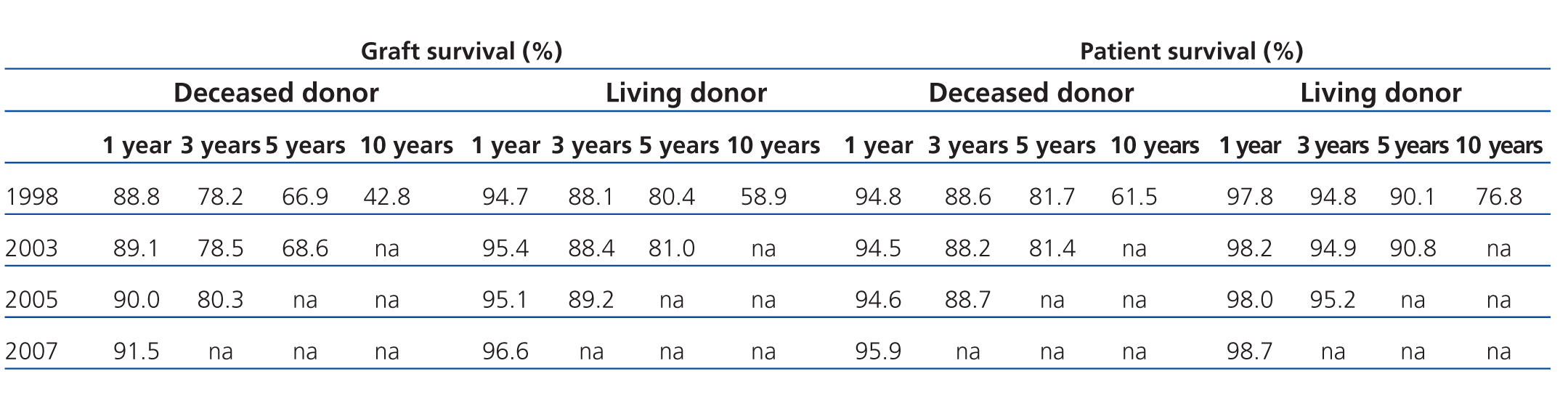
However, the decrease in acute rejection rates has not been associated with increased long-term graft survival.5 Results from the OPTN/SRTR 2009 report showed no significant differences over time (Table 1).3 As such, strategies are still being sought that can increase long-term graft survival and patient survival.
Chronic graft dysfunction continues to be a common cause of graft loss. In the Spanish ICEBERG epidemiological study, 55.5% of patients developed chronic allograft nephropathy during a follow-up period of 8 years, average.6 In a Spanish study involving 1029 kidney recipients between 1997 and 2007, no significant differences in graft survival were observed after 5 and 10 years when only analysing cases where the graft had already survived more than 12 months.7 In the second phase of the study by the Spanish Chronic Allograft Nephropathy Study Group, patients that received a kidney transplant in 2002 were also included.8 Twelve months after the transplant, mean estimated glomerular filtration rate (eGFR) was 51.7±18.8ml/min/1.73m2. During the follow-up period that lasted a mean 74.0±43.9 months, eGFR decreased by a mean 1.6±6.24ml/min/year. This decrease was more pronounced in patients treated with cyclosporine (n=3163) than those treated with tacrolimus (n=1044) and the difference was even greater with respect to those not receiving calcineurin inhibitors (n=133; drugs not specified).9
Cardiovascular disease following a kidney transplant was researched by the Spanish group Forum Renal, which created a prospective, multi-centre database of 2600 kidney transplantations during the period of 2000-2002. The rate of acute rejection at 12 months was 14.8%. After 4 years, graft and patient survival were 85.6% and 91.7%, respectively. The leading cause of death was cardiovascular disease, mainly coronary disease during the first year. The primary causes of graft loss were: vascular disease and thrombosis during the first year, death with a functioning graft in the second and third years, and chronic allograft nephropathy in the fourth year.10
In addition to chronic allograft nephropathy and cardiovascular morbidity and mortality rates, infectious morbidity and mortality,11 metabolic complications,12 and a high frequency of tumours all impede long-term success.13
The primary interest in developing new immunosuppressants no longer involves simply improving short-term results, but also improving the safety profile, lowering nephrotoxicity, and improving cardiovascular and metabolic profiles.14 Furthermore, the tolerability and adverse effects of immunosuppressants are just as important as their efficacy, above all when taking into account that kidney transplant recipients are growing older, with associated cardiovascular comorbidities.15
NEW IMMUNOSUPPRESSANTS FOR KIDNEY TRANSPLANTS
The currently used immunosuppressant regimens are designed to block the activation, proliferation, and functioning of T cells (TC).16 The activation and proliferation of TC depends on three signals. The first is given by the interaction between the TC receptor and class II molecules, expressed in antigen presenting cells (APC). Calcineurin is activated at this point, and this is where calcineurin inhibitors, such as cyclosporine and tacrolimus, come into play. The second signal, or costimulatory signal, depends on the interaction of CD80/86 in the APC and CD28 expressed in the TC surface. Belatacept inhibits this interaction. The first and second signals activate transduction pathways that generate transcription factors for the synthesis of cytokines. Among these, interleukin 2 (IL-2) stands out, as it induces the third signal, allowing for the proliferation of TC clones (this is where immunosuppressants that interfere with the cell cycle play a role). Several strategies have been developed for improving kidney transplant results using traditional immunosuppressants,13,17-26 but the rates of chronic transplant nephropathy and cardiovascular and metabolic risk oblige us to continue seeking out alternatives.
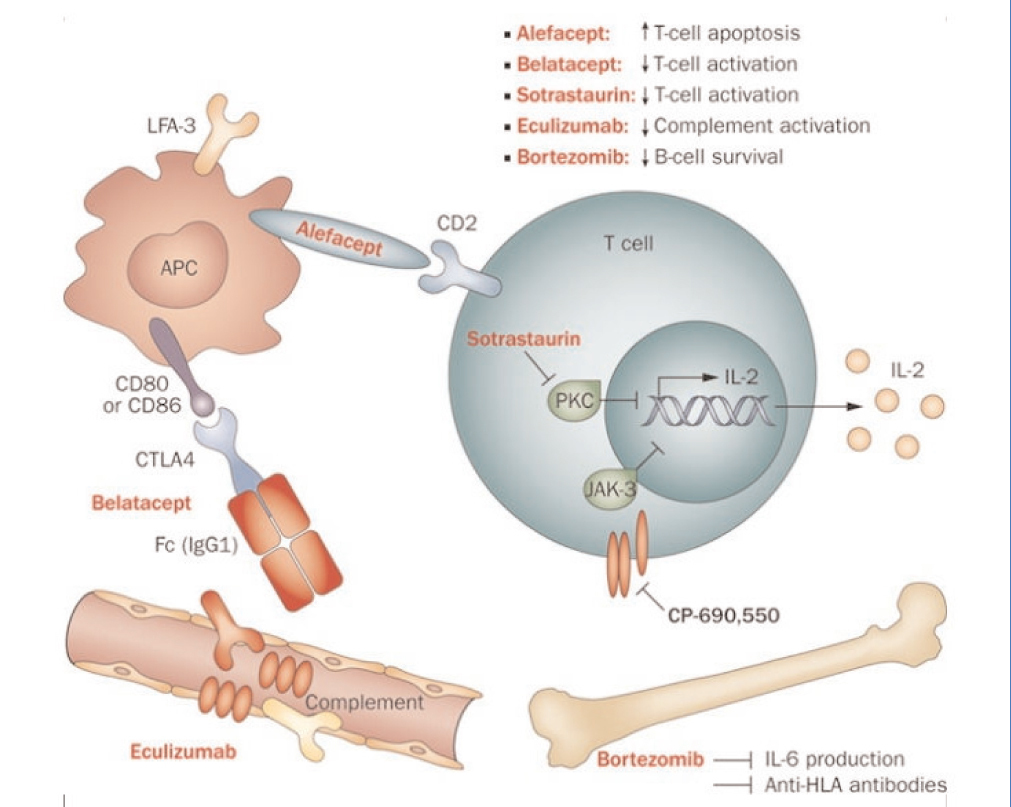
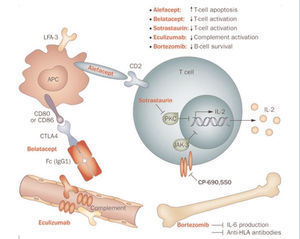
Several different drugs and biological agents are being evaluated in clinical trials or have recently been approved for immunosuppression in kidney transplants. These new immunosuppressants have different mechanisms of action. Some inhibit enzymes such as protein kinase C (PKC) or Janus kinase 3 (JAK3), and others act on key components of the lymphocyte activation process, such as the LFA3-CD2 or CD28-CD80/86 pathways (Figure 1).27
Sotrastaurin is a potent and selective inhibitor of classic (α and β) and new (δ, ϵ, η, and θ) isoforms of PKC. These isoforms play a key role in the first and second signals for TC activation. By inhibiting these isoforms, sotrastaurin blocks the initial activation of T cells, although it does not affect the lymphocyte proliferation that is mediated by IL-2.28 Two phase II trials were interrupted due to an increase in acute rejection.14,29 In another phase II trial, sotrastaurin was compared to tacrolimus in 125 patients that received a kidney transplant (de novo recipients). After 3 months, sotrastaurin was significantly less effective than tacrolimus using the primary evaluation criteria (acute rejection confirmed by biopsy, graft loss, death, or loss to follow-up), 25.7% vs 4.5% (P=.001). The difference in acute rejection rates, 23.6% with sotrastaurin compared to 4.5% with tacrolimus (P=.003) caused the interruption of the study.30
CP-690550 or tofacitinib (previously referred to as tasocitinib) is a selective inhibitor of JAK3 kinase which plays a central role in signal transduction for cytokine receptors.31 In phase II trials, tofacitinib has demonstrated comparable efficacy to tacrolimus in terms of acute rejection and renal function.14,32 In a pilot trial, the lower dose of CP-690550 had similar safety and efficacy profile to tacrolimus in kidney transplant recipients.33 Later, a multi-centre, randomised study was carried out in which 322 kidney transplant recipients were randomised to receive CP-690550 or cyclosporine for 12 months. The incidence of acute rejection was similar in both groups, although severe infections were more common with CP-690550 than with cyclosporin.34 However, eGFR improved with CP-690550 from the 1st month, and the effect was maintained throughout the study, with significantly different results from cyclosporine (P<.05 for both regimens of CP-690550).35
Alefacept is a humanised anti-CD2 monoclonal antibody. It is a LFA3-IgG1 fusion protein that inhibits the adhesion of T cells. The results of alefacept in kidney transplants in primates were very promising,36 which led to further phase I and II trials, administering the drug orally along with tacrolimus in kidney transplants. However, the results from a randomised, double-blind, placebo controlled, multi-centre, phase II trial with 212 kidney transplant recipients did not confirm these expectations. Alefacept did not result in significantly superior outcomes than the placebo, except for in the activation of CD4+ and CD8+ T cells, with no differences in graft survival, patient survival, or renal function.37
BELATACEPT
Belatacept is a selective T-cell blocker. It is a human fusion protein that combines a modified extracellular portion of cytotoxic T-cell antigen 4 (CTLA-4) and the fragment crystallisable region of human IgG1 (Fc region). It blocks the costimulatory signal by binding to APC CD80 and CD86 antigens, thus inhibiting the complete activation of T cells and promoting anergy and apoptosis. This drug is derived from abatacept, a fusion protein that is effective in autoimmune disorders such as rheumatoid arthritis. The belatacept molecule includes two replaced amino acids that confer a greater binding ability to CD80 and CD86, greater binding strength to T cells, and greater efficacy in prophylaxis against rejection.38
In June 2011, the European Medicines Agency (EMA) approved belatacept for combined use with steroids and mycophenolic acid in prophylaxis against graft rejection in adult patients that had received a kidney transplant.39 This is the first biological agent to be approved for this indication, and is the first immunosuppressant in a decade to offer a new mechanism of action.
Belatacept has linear pharmacokinetics in healthy volunteers and kidney transplant recipients. Patient exposure to the drug is proportional to dosage, with very little day to day variability. Drug levels in the body are predictable based on the doses administered intravenously, regardless of sex, age, race, renal function, albuminemia, diabetes, and dialysis treatment.40 In clinical trials, the minimum concentration was maintained stably up to 5 years following the transplant.41
The saturation of CD 86 receptors inhibit the T-cell alloresponse. In vitro studies have demonstrated alloresponse inhibition with the minimum concentration of belatacept necessary to saturate CD86 receptors. In patients treated with belatacept, free CD86 receptor levels are significantly lower than before treatment, and are also lower than in healthy volunteers and patients treated with cyclosporin.42 The extension to 5 years of a phase II trial involving patients treated with belatacept or cyclosporine, CD86 receptor saturation with belatacept was maintained throughout the follow-up period. However, belatacept saturation in patients treated every 4 weeks (74%) was significantly higher than in patients treated with belatacept every 8 weeks (56%) (P<.05),41 confirming that saturation of CD86 receptors depends on the frequency and dosage of belatacept administered.
Phase II trials
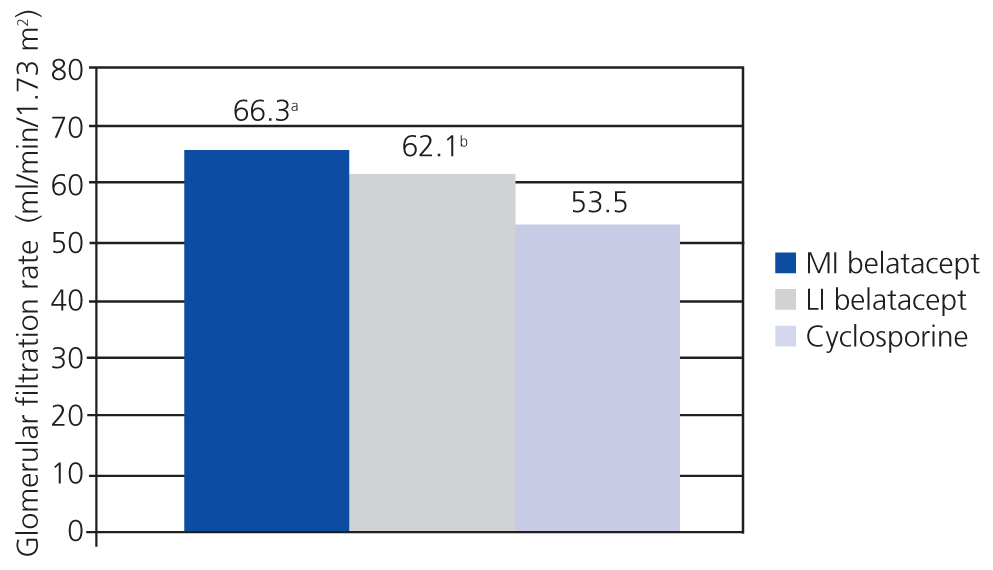
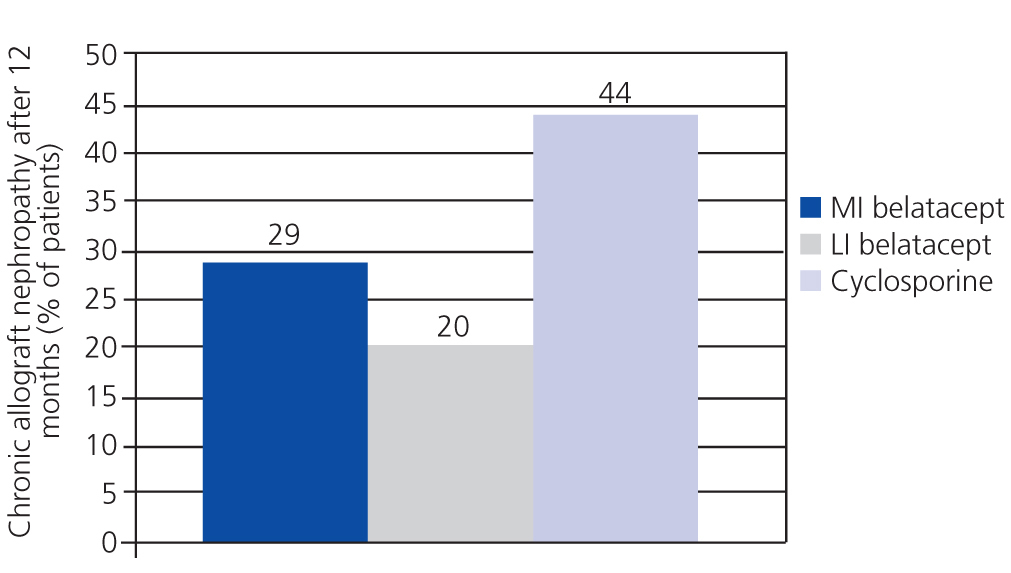
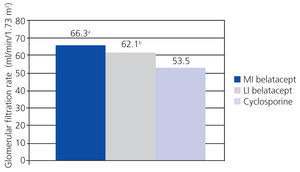
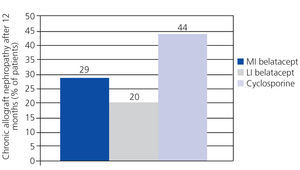
A randomised, partially blinded study with parallel groups carried out in 22 centres in Europe, USA, and Canada, and involving 216 patients, compared belatacept administered in more intensive (MI) or less intensive (LI) treatment schedules vs cyclosporine in the prevention of rejection within 6 months of transplantation.38 The incidence of acute rejection within 6 months was similar between the three groups: 7% with MI belatacept, 6% with LI belatacept, and 8% with cyclosporine. There were no cases of rejection after 6 months. Directly measured GFR was higher with belatacept after 12 months (Figure 2). The best correlations between GFR and eGFR were achieved using the Modification of Diet in Renal Disease (MDRD) equation. Chronic allograft nephropathy was less frequent with belatacept (Figure 3). Patients with chronic allograft nephropathy that received belatacept had a higher eGFR than the group that received cyclosporine. In terms of cardiovascular and metabolic profiles, blood pressure, and lipid levels in patients treated with belatacept were similar or slightly lower than in the group treated with cyclosporine, despite the greater use of anti-hypertensive and lipid-lowering drugs in this group.
This study demonstrated that belatacept was not inferior to cyclosporine, and even suggested that belatacept could preserve GFR and reduce the incidence of chronic allograft nephropathy.38
In order to evaluate the long-term efficacy and safety of belatacept, 102 patients treated with belatacept (90%) and 26 treated with cyclosporine (51%) from the previous study completed 60 months of treatment.41 The percentages of participation show good acceptance of belatacept. Renal function remained stable throughout the study in patients treated with belatacept, with an eGFR (MDRD) of 75.8±20.1ml/min/1.73m2 12 months after the transplant, and 77.2±22.7ml/min/1.73m2 after 5 years. In contrast, eGFR decreased in the group treated with cyclosporine (74.4±23.7ml/min/1.73m2 after 12 months and 59.3±15.3ml/min/1.73m2 after 5 years).
As regards cardiovascular risk factors, there was a slight increase in systolic and diastolic blood pressure between months 24 and 60 in patients treated with cyclosporine, with final values of 138±18.9mm Hg and 83±8.9mm Hg, respectively, compared to 125±13.9mm Hg and 76±10.1mm Hg with belatacept (note: no P-values are available, data extracted from Table 1 in Vincenti, 2010). The levels of non-HDL cholesterol decreased in both groups, although the use of lipid-lowering drugs was lower in patients treated with belatacept. When the study period was extended to a long-term period, the frequency of new-onset diabetes after transplantation (NODAT) was similar in the two groups.
The incidence rates of death/graft loss and acute rejection were low. The incidence of severe infections was 16% with belatacept and 27% with cyclosporine. Neoplasia occurred in 12% of patients in both groups, with only one case of post-transplant lymphoproliferative disorder (PTLD) in the cyclosporine group and none in the belatacept group. Coronary disease was more common in the cyclosporine group (12% vs 2% with belatacept).
The study demonstrated good compliance with treatment, stable renal function, a high level of safety, and predictable pharmacokinetics with belatacept during a 5-year follow-up period. No new cases of PTLD occurred with belatacept.41
In an open, randomised, controlled phase II trial, kidney transplant recipients were treated with belatacept/MMF (n=33), belatacept/sirolimus (n=26), or tacrolimus/MMF (n=30) for 12 months. The incidence of acute rejection after 6 months was 12% with belatacept/MMF, 4% with belatacept/sirolimus, and 3% with tacrolimus/MMF. Renal function was better with the two different belatacept regimens, with a mean eGFR (MDRD) that was 8-10ml/min/1.73m2 higher than in the tacrolimus/MMF group. There were no significant differences in terms of safety, including cardiovascular risk profile.43
This same study demonstrated a potent inhibitory activity in the combination of belatacept/sirolimus, which can be as effective as a calcineurin inhibitor (CNI) in halting the antigen-specific and T-cell response. In the first year of the study, the percentage of regulatory T cells (Treg) in the memory T-cell compartment was significantly higher in patients treated with belatacept/sirolimus. The combination of depleted T cells, costimulatory blockade, and mTOR inhibition appears to be effective in preserving Treg and inhibiting immune responses.44
Another open and randomised phase II trial compared the replacement of a CNI (cyclosporine or tacrolimus) with belatacept against continuing treatment with the CNI in 175 patients that had received a kidney transplant 6-36 months ago and had stable graft function. After 12 months, 6 patients that had converted to belatacept had developed acute rejection, but no graft losses occurred. Graft survival was 100% in the group treated with belatacept and 99% in the group treated with a CNI. Belatacept produced a mean increase in eGFR (MDRD) of 7.0±11.99ml/min/1.73m2, whereas the mean increase with a CNI was 2.1±10.34ml/min/1.73m2 (P=.0058). The switch from a CNI to belatacept improved renal function and was associated with a low rate of acute rejection.45
In order to evaluate whether the benefits of belatacept were maintained over long periods, the follow-up protocol was expanded to 2 years in 162 patients from the previous study. Mean eGFR was higher with belatacept (62.0ml/min/1.73m2) than with CNI (55.4ml/min/1.73m2). The mean change in eGFR from the start of the study was much higher with belatacept (8.8ml/min/1.73m2) than with calcineurin inhibitors (0.3ml/min/1.73m2). The benefit of belatacept on renal function was observed in patients previously treated with cyclosporine (7.8ml/min/1.73m2) or tacrolimus (8.9ml/min/1.73m2), and it was independent of renal function at the start of the study. There were also differences in the incidence of acute rejection; rejection occurred in 4.9% of the cases treated with belatacept, and only during the first year, whereas the rate was 3.7% with CNI, all cases occurring in the second year. The safety profile was similar for all groups, and no cases of PTLD were observed.46
Phase III trials
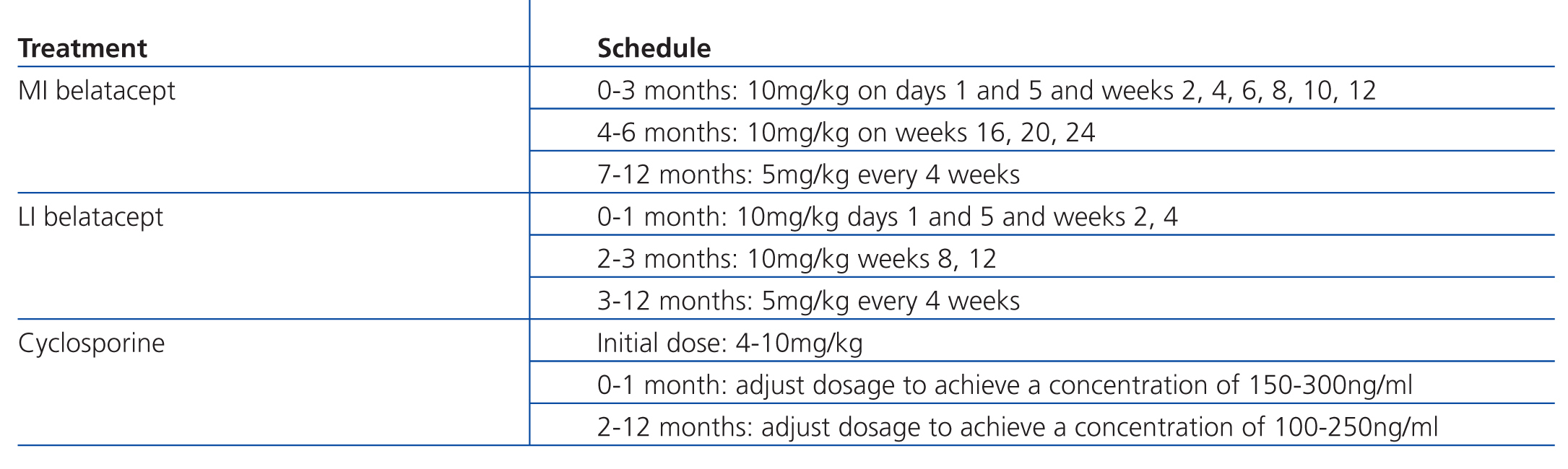
In the international BENEFIT study (Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial), MI and LI regimens of belatacept were compared to cyclosporine (Table 1) as maintenance immunosuppression therapy in 666 kidney recipients. The primary evaluation criteria were patient and transplant survival, along with a composite endpoint involving renal deterioration and incidence of acute rejection. After 12 months, patient/graft survival was similar in the three treatment groups (95%, 97%, and 93%, respectively). However, renal function was significantly better with both belatacept regimens. The composite endpoint of renal deterioration was reached in 55% of MI belatacept patients and 54% of LI belatacept patients, as compared to 78% of patients with cyclosporine (P≤.001 for MI or LI vs cyclosporine). Mean eGFR was 65ml/min/1.73m2 and 63ml/min/1.73m2 for MI and LI belatacept, respectively, whereas it was 50ml/min/1.73m2 for cyclosporine (P≤.001 for MI or LI vs cyclosporine). eGFR was calculated using the MDRD equation. Acute rejections were more common with belatacept (22% MI and 17% LI) than with cyclosporine (7%). Safety was similar with both drugs, although more cases of PTLD occurred with belatacept.47
In the phase III BENEFIT-EXT study (Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial-EXTended criteria donors), 543 kidney transplant recipients were evaluated that had received kidneys from expanded criteria donors. The primary evaluation criteria were the same as in the BENEFIT study. There were no significant differences in patient/graft survival: 71% with MI, 77% with LI, and 85% with cyclosporine (P=.002 for MI vs cyclosporine and P=.06 for LI vs cyclosporine). Renal function was significantly better with belatacept, with an eGFR that was 4-7ml/min/1.73m2 higher (P=.008 for MI vs cyclosporine and P=.1309 for LI vs cyclosporine). Cardiovascular and metabolic profiles were better with belatacept. The incidence of acute rejection was similar between groups (18% for MI and LI and 14% for cyclosporine).48
The combined data analysis from the BENEFIT and BENEFIT-EXT studies after 2 years of treatment included 840 patients. The proportion of surviving patients with a functioning graft was similar between all treatment groups. Belatacept continued to provide superior results to cyclosporine in terms of renal function, with an eGFR that was a mean 16-17ml/min/1.73m2 higher in the BENEFIT study and 8-10ml/min/1.73m2 higher in the BENEFIT-EXT study as compared to cyclosporine. Very few episodes of acute rejection occurred in the second year of the trials. Cardiovascular and metabolic profiles were better with belatacept than with cyclosporine. The incidence of PTLD is greater in patients with negative serology tests for Epstein-Barr virus (EBV-), and so the efficacy of this drug was specifically analysed in EBV+ patients, with coinciding results with those from the overall study population. No new adverse effects were observed. The authors concluded that the LI regimen of belatacept was preferable to the MI regimen, since it provided a better balance between efficacy and safety.49
The 3-year results from both trials showed that belatacept maintains its effects on a long-term basis, with high patient and graft survival rates,50 even in recipients of kidneys from expanded criteria donors.51
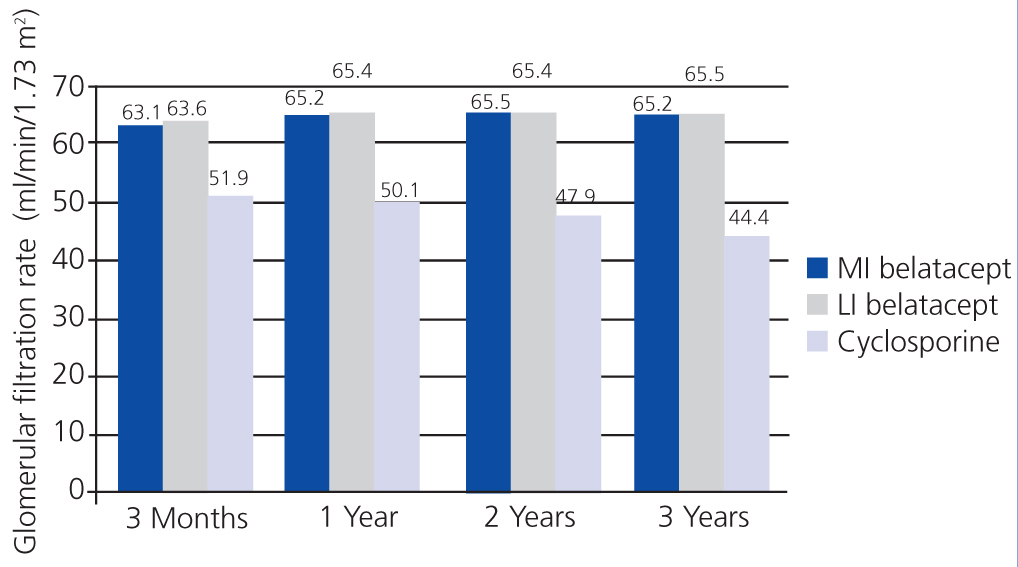
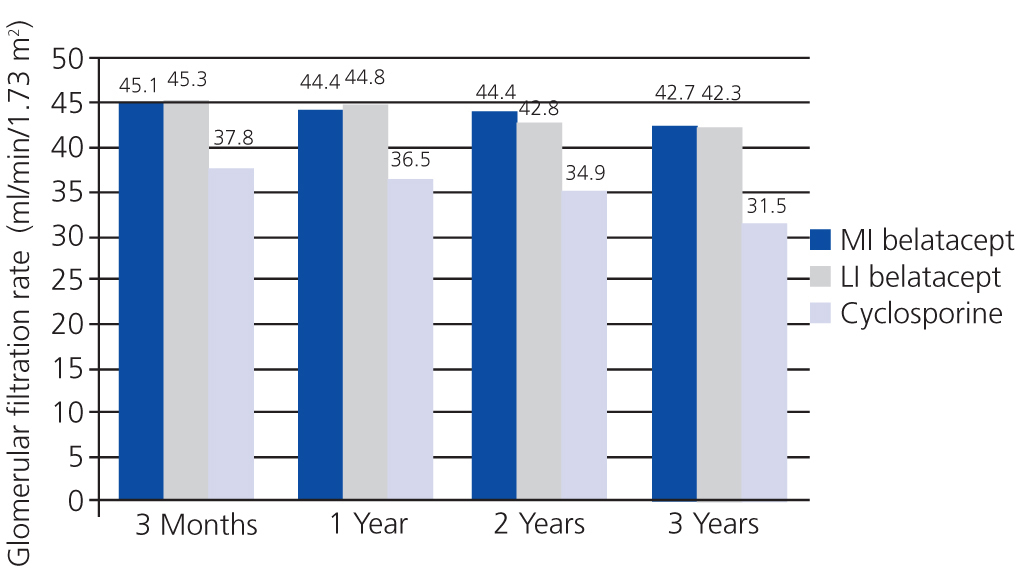
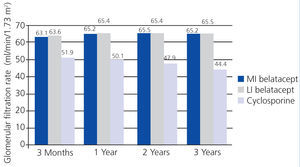
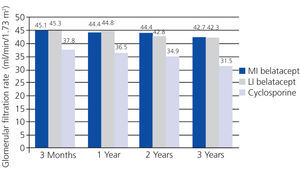
In the BENEFIT study, renal function was better with belatacept than with cyclosporine from the third month until the end of the three-year study. Treatment with belatacept was associated with a greater probability of improved renal function.52 In the combined analysis of renal function results from the BENEFIT and BENEFIT-EXT studies, belatacept was superior (Figure 4 and Figure 5). Change in mean eGFR (ml/min/year) between months 3 and 36 was 1.0 (MI belatacept), 1.2 (LI belatacept), and -2.0 (cyclosporine) in the BENEFIT study, and -0.9, -0.6, and -1.9, respectively, for the BENEFIT-EXT study. Advanced chronic renal failure (eGFR<30ml/min) was more common in patients treated with cyclosporine in both studies: 20% in the BENEFIT study and 44% in the BENEFIT-EXT study as compared to MI or LI belatacept: 9% and 10% in the BENEFIT study and 27% and 30% in the BENEFIT-EXT study.53 In another analysis, renal function results were compared by donor type (deceased or living donor), with no significant differences observed in GFR improvement amongst patients treated with belatacept.54
Safety of belatacept
Clinical studies have demonstrated good tolerance with belatacept. The safety profile of belatacept was evaluated in an analysis of the pooled data from the three key belatacept studies in kidney recipients.38,47,48 The data for 1425 patients were analysed, with a mean follow-up time of 2.4 years. With LI belatacept, the death rate (5%) was lower than with cyclosporine or MI belatacept (7% each), which also occurred with the rate of neoplasia (32% vs 36% and 37%, respectively). The rate of infection was similar between patients treated with belatacept and with cyclosporine. One case of progressive multifocal leukoencephalopathy was observed in a patient treated with belatacept at higher doses than recommended, and who was also receiving IL-2 receptor antagonists, MMF, and steroids. The tolerance to the infusion was good, which facilitated compliance with the treatment protocol, and no cases of anaphylaxis or hypersensitivity were reported. According to the results from this analysis, the LI regimen is preferable to the MI,55 since it provides a better safety profile that MI and the same efficacy.
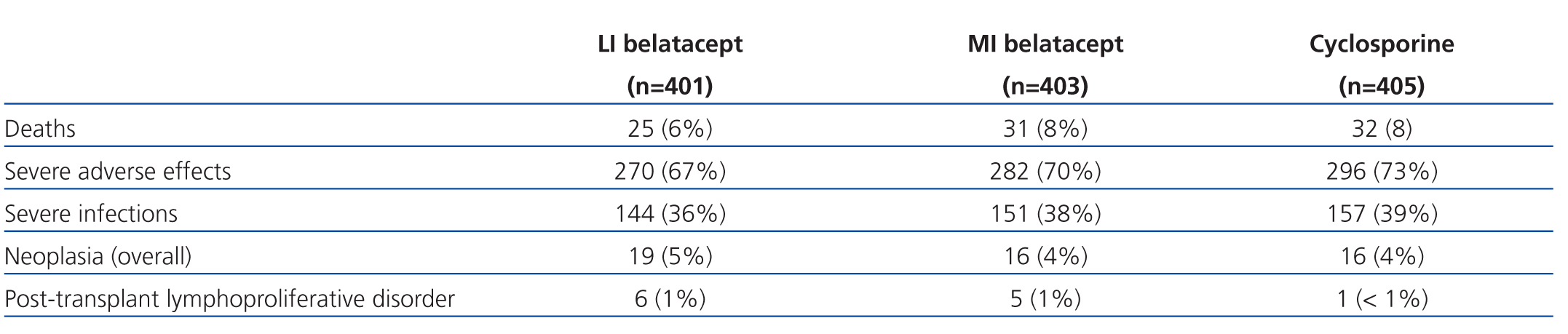
The safety profile analysis for belatacept after 3 years of treatment in the BENEFIT and BENEFIT-EXT studies included the data from 1209 patients. LI belatacept was associated with fewer deaths and cases of severe infection than MI belatacept and cyclosporine (Table 3).56 The risk of PTLD with belatacept (1% for each treatment schedule) was within the expected incidence rate following a kidney transplant,57 and decreased after 18 months.
Cases of PTLD have arisen in phase II and III trials. There were 16 cases of PTLD: 8 under the MI regimen (2%), 6 under the LI regimen (1%), and 2 with cyclosporine (0.4%), 9 cases having central nervous system (CNS) problems among those treated with belatacept (6 with MI and 3 with LI).55 The risk for developing this condition was greater in the first 18 months of treatment, in patients on the MI belatacept regimen and in those with EBV- serology at the time of transplant. In a later analysis of biopsies by a central pathologist, two of the cases described as graft PTLD, both in LI patients, were later diagnosed as acute rejection in one case and non-specific T-cell proliferation in the other.55
Calcineurin inhibitors increase the risk of infection by cytomegalovirus (CMV) by inhibiting specific memory T cells. Results from in vitro studies and seropositive volunteers for CMV suggest that belatacept protects against transplant rejection, but does not alter the response of CMV-specific memory T cells. As a result, belatacept does not increase the risk of infection by CMV,58 which is known to be a risk factor for developing PTLD when coexisting with an EBV infection.
The cardiovascular risk profile for belatacept could be more favourable than currently used immunosuppressants. In the BENEFIT and BENEFIT-EXT trials, belatacept was superior to cyclosporine in the parameters evaluated.59 Mean systolic blood pressure was 6-9mm Hg lower, and mean diastolic blood pressure was 3-4mm Hg lower in patients treated with belatacept than in those treated with cyclosporine (P≤.002).
Non-HDL cholesterol levels were lower with belatacept (P<.01 with MI or LI belatacept compared to cyclosporine in both studies), and a similar trend occurred in serum triglycerides (P<.02 with MI or LI belatacept compared to cyclosporine in both studies).
In prespecified pooled analysis, NODAT was significantly less frequent with MI or LI belatacept (5%) than with cyclosporine (10%) (P<.05 for MI or LI belatacept compared to cyclosporine). The results from the BENEFIT and BENEFIT-EXT trials showed that belatacept provides a better cardiovascular and metabolic profile than cyclosporin.59
In one of the phase II trials, blood samples were taken at least every 12 months before the belatacept infusion. The samples were analysed in a sensitive electrochemiluminescence immunoassay that was validated for the detection of anti-belatacept antibodies directed against the whole molecule or the modified CTLA-4 portion of belatacept. This type of antibody was detected in six patients treated with the 4-week treatment regimen and in ten patients treated with the 8-week regimen, although only two of the patients in the first group and six in the second still had positive test results on the last follow-up check. Two of the patients from the 8-week group developed neutralising antibodies, but both continued treatment. None of the patients with anti-belatacept antibodies had graft loss or acute graft rejection, and none died or suffered severe adverse autoimmune side effects or conditions related to the infusion.41
In contrast to other immunosuppressants used as the background treatment in organ transplantation, belatacept does not require monitoring of drug levels in the body, since it does not have a narrow therapeutic range.
The risk of interaction with other drugs is very low, since, in contrast with other immunosuppressants used in transplants, belatacept is a fusion protein that is not metabolised by cytochrome P450 enzymes (CYP) or UDP-glucuronosyltransferase.40
COMMENTS
Belatacept is the first drug in a new class of immunosuppressants. The data from clinical trials comparing belatacept to cyclosporine suggest that they have similar efficacy, but belatacept preserves renal graft structure and function, and is associated with lower rates of chronic allograft nephropathy. In long-term treatment regimens, renal function remained stable, which contrasts with the annual decrease of 1-3ml/min/m2 that is usually observed with calcineurin inhibitors at stable doses, and is consistent with 1st year results.41,60 The recommended initial dose is 10mg/kg on days 1 (before the intervention), 5, 14, and 28, and after weeks 8 and 12 following the transplantation. In the maintenance phase, the recommended dose is 5mg/kg every 4 weeks (±3 days), starting at the end of the 16th week following the transplant.39
Belatacept offers a more favourable cardiovascular and metabolic profile than calcineurin inhibitors. According to the results from a systematic review of randomised and controlled studies, patients treated with belatacept had a 69% lower probability of death, compared to those treated with tacrolimus.61 Cardiovascular disease is the most common cause of death in patients with kidney transplants and functioning grafts. In clinical trials such as BENEFIT and BENEFIT-EXT, the incidence of NODAT with belatacept has been lower than in patients treated with calcineurin inhibitors, and belatacept has demonstrated a better cardiovascular and metabolic profile than currently used immunosuppressants. In summary, belatacept can provide a better cardiovascular and metabolic profile than current immunosuppressants.
Kidney transplants from expanded criteria donors are becoming more and more frequent due to the increased demand for organs. However, the risk of graft loss or dysfunction is higher in these donors, who tend to be older and have associated morbidity. Graft survival after one year is lower than in normal organ donors, and survival in later years is even lower, mainly due to chronic allograft nephropathy.62,63 The BENEFIT-EXT study demonstrated that belatacept is effective in recipients of kidneys from expanded criteria donors, and its use in this context could prevent nephrotoxicity associated with calcineurin inhibitors.41
In clinical trials, the primary risk of treatment with belatacept has been PTLD, especially in the first 18 months of treatment. A relationship has been suggested between the intensity of immunosuppression and PTLD, since patients treated with MI belatacept suffered from CNS-related events more frequently and had a higher risk of CNS infections. It was proposed that the risk of PTLD could be reduced by administering the LI regimen of belatacept and avoiding treatment in EBV- patients or those with unknown serology,49 since the primary risk factor is an EBV- serology but CMV infection and T-cell depletion therapy also increases the risk. Currently, the technical data sheet for belatacept states that it is contraindicated in patients with EBV- or unknown serology.64
Since CNS involvement in PTLD is more common with belatacept than cyclosporine, we must keep this possibility in mind when treating kidney recipients with belatacept who develop neurological, cognitive, or behavioural symptoms.64
The use of belatacept as the background therapy in kidney recipients preserves renal function and is associated with a lower incidence of cardiovascular risk factors and NODAT. The inclusion of belatacept in immunosuppression protocols for kidney transplants could facilitate a major improvement in patient and graft survival.
Key points
- Despite the availability of useful and effective immunosuppressants for the prevention of acute kidney transplant rejection, long-term results are still unsatisfactory, and new immunosuppressant treatments are needed that provide better efficacy and safety.
- Belatacept is an immunosuppressant with a selective mechanism of action, whose primary characteristics are:
- Improved renal function that is maintained over time;
- Cardiovascular and metabolic risk profiles are much more favourable than with calcineurin inhibitors;
- Good tolerability;
- No infusion reactions;
- Contraindicated in transplant recipients with EBV negative or unknown serology.
Acknowledgements
The authors would like to thank Content Ed Net Communications for their editorial assistance.
Table 1. Evolution of graft and patient survival between 1998 and 2007, according to the OPTN/SRTR 2009 report
Table 2. Treatments in the BENEFIT study
Table 3. Safety profile in the BENEFIT and BENEFIT-EXT studies
Figure 1. Intracellular signalling and inhibition by new compounds
Figure 2. Both treatment schedules of belatacept were significantly superior to cyclosporine in terms of renal function
Figure 3. Chronic allograft nephropathy was less frequent with both treatment schedules of belatacept
Figure 4. Glomerular filtration rates when using belatacept and cyclosporine during the BENEFIT study
Figure 5. Glomerular filtration rates when using belatacept and cyclosporine during the BENEFIT-EXT study