Reflexionamos sobre las discrepancias encontradas en el uso generalizado de metformina en pacientes con diabetes mellitus tipo 2, la ausencia de criterios uniformes acerca de su indicación en los diferentes estadios de insuficiencia renal y sobre su empleo en estos pacientes. Realizamos un corte transversal en 304 pacientes diabéticos tipo 2, visitados a lo largo de 2010 de forma consecutiva en consulta de Atención Primaria, Endocrinología y Nefrología, con una tasa de filtrado glomerular (TFG) estimada < 60 ml/min/1,73 m2 y tratados con antidiabéticos orales (ADO). Revisamos la frecuencia de uso de metformina y otros antidiabéticos en función del tipo de consulta y el grado de función renal. El ADO más utilizado fue metformina (54,9%), seguido de repaglinida (47,7%), dipeptidil-peptidasa (IDPP-4) (28,6%) y sulfonilureas (18,4%). Observamos menor uso de metformina y mayor de repaglinida, estadísticamente significativo, en pacientes de Nefrología, y mayor de IDPP-4 en Atención Primaria. La metformina fue la menos utilizada, con TFG entre 29-15 ml/min/1,73 m2 (13,3%), junto con las sulfonilureas, y la más prescrita en TFG mayores (70,0% con 59-45 ml/min/1,73 m2), p < 0,001. La repaglinida fue más utilizada, con TFG entre 29-15 ml/min/1,73 m2 (76,7%), mientras que se prescribió menos con TFG mayores (38,9% con 59-45 ml/min/1,73 m2), p < 0,001. En nuestra opinión, en la literatura existen evidencias sobre el uso de metformina en pacientes con TFG entre 30-60 ml/min/1,73 m2 que permiten sugerir su empleo con precaución en este grupo de pacientes y, algo que es importante para la práctica médica, hacerlo dentro de un marco legal.
In this paper we analyse the discrepancies that exist in the widespread prescription of metformin in patients with type 2 diabetes and the lack of guidelines concerning its prescription in the different stages of renal failure. This cross-sectional study includes 304 patients with type 2 diabetes treated with oral antidiabetic drugs (ADOs) and a glomerular filtration rate (estimated GFR) <60ml/min/1.73m2. Patients were attended in consecutive visits to primary health centres or in hospital departments of endocrinology or nephrology during 2010. We studied the frequency of metformin and other ADO prescriptions according to renal function and the department in which the patient was treated. The ADO most frequently prescribed was metformin (54.9%), followed by repaglinide (47.7%), DPP4 inhibitors (28.6%), and sulfonylureas (18.4%). However, in nephrology departments, repaglinide was more frequently prescribed than metformin (P<.001), whereas in primary health centres, the prescription of DPP4 inhibitors increased. In patients with an estimated GFR of 15-29ml/min/1.73m2, metformin (13.3%) and sulfonylureas were the least prescribed, whereas metformin was much more frequently prescribed (70.0%) when estimated GFR was 45-59ml/min/1.73m2 (P<.001). In contrast, patients with an estimated GFR of 15-29ml/min/1.73m2 were mainly prescribed repaglinide (76.7%), as opposed to patients with an estimated GFR of 45-59ml/min/1.73m2 (38.9%) (P<.001). Substantial evidence suggests that the recommendations for the use of ADO should be modified. This would lead to safely prescribing ADO in patients with an estimated GFR<60ml/min/1.73m2, and more importantly in medical practice, according to the law.
INTRODUCTION
There is a complex arsenal of therapeutic options for the treatment of patients with type 2 diabetes mellitus (DM), including metformin, sulfonylureas, glinides, thiazolidinediones, disaccharidase inhibitors, dipeptidyl peptidase (DPP-4) inhibitors, and glucagon-like peptide-1 (GLP1) receptor antagonists, which, along with insulin, can be used in monotherapy or combined treatment. These drugs must be used after careful consideration of their technical data sheets. The choice depends on several different inter-related patient aspects, the ability of the drug to achieve treatment targets, associated diseases and complications, the risk of adverse effects, tolerance, and cost.1
The main national (Spanish Society of Diabetes [SED]1) and international2-5 consensus documents and guidelines for the treatment of type 2 DM recommend using metformin as the first line of treatment, along with hygienic and dietary modifications, from the moment a diagnosis of DM is confirmed. However, there are no standard criteria for its use in the different stages of renal failure.
The SED1 and the American Association of Clinical Endocrinologists/American College of Endocrinology Consensus Panel on Type 2 DM (AACE/ACE) contraindicate the use of metformin in patients with a glomerular filtration rate (GFR) <60ml/min/1.73m2, whereas Canadian and Australian guidelines place the cut-off point at GFR<30ml/min/1.73m2, and recommend caution in prescribing this drug in patients with GFR<60ml/min/1.73m2, which is in agreement with the consensus document from the American Diabetes Association and the European Society for the Study of Diabetes.3
The results from the UKPDS study already demonstrated the capacity of metformin to reduce glycaemia and the risk of micro and macroangiopathic complications in overweight patients.6 Additionally, metformin presents a series of advantages that provide an added value: it does not induce hypoglycaemia,7 has a neutral impact (or slight decrease) on body weight,7 improves lipid profiles,7,8 and also improves insulin resistance,7 all while maintaining a low cost. The limitations for its use are primarily derived from digestive intolerance, renal failure, liver failure, and acute/chronic pathologies that may cause tissue hypoxia.9
The aim of our study was to analyse the prescription of oral antidiabetics (ADO), especially metformin, which is the most commonly used ADO, by a group of health professionals from different specialties (primary care, endocrinology, and nephrology) in patients with renal failure and a MDRD-4 (Modification of Diet in Renal Disease-410) estimated GFR<60ml/min/1.73m2, as they do not fall within the technical data sheet indications, the legal document that serves as the basis for drug prescription.11
MATERIAL AND METHOD
We performed a cross-sectional study of patients diagnosed with type 2 DM in consecutive visits during 2010 in primary care, endocrinology, and nephrology departments. Patients were only included in the study with an estimated GFR<60ml/min/1.73m2, according to laboratory results, and who were receiving treatment with ADO. The variables collected were age, sex, last serum creatinine measurement (mg/dl), GFR calculated using the MDRD-4 formula, and latest measurements of glycosylated haemoglobin (HbA1c) and albuminuria/proteinuria. We also recorded all antidiabetic drugs prescribed: metformin, sulfonylureas, DPP-4 inhibitors, and repaglinide. The possible concomitant use of insulin and treatment compliance were also taken into account.
Quantitative variables are expressed as mean ± SE, and were compared using Student’s t-tests or Mann-Whitney U-tests, based on their distribution. Categorical variables were analysed using chi-square tests. A P-value <.05 was considered statistically significant, and we used G-Stat statistical software, version 2.0, for the analyses.
RESULTS
We analysed a total of 304 patients diagnosed with type 2 DM and treated with ADO, all of which had a GFR<60ml/min/1.73m2 (MDRD-4) and a mean age of 74.2±9.0 years; of these, 128 were male (42.1%) and 176 were female (57.9%). Mean creatinine was 1.42±0.48mg/dl (range: 0.90-3.67mg/dl), with a mean GFR (MDRD-4) of 45.5±11.1ml/min/1.73m2. Some 180 patients (59.2%) had a GFR of 45-59ml/min/1.73m2, 94 (30.9%) had a GFR of 30-44ml/min/1.73m2, and 30 (9.9%) had a GFR of 15-29ml/min/1.73m2.
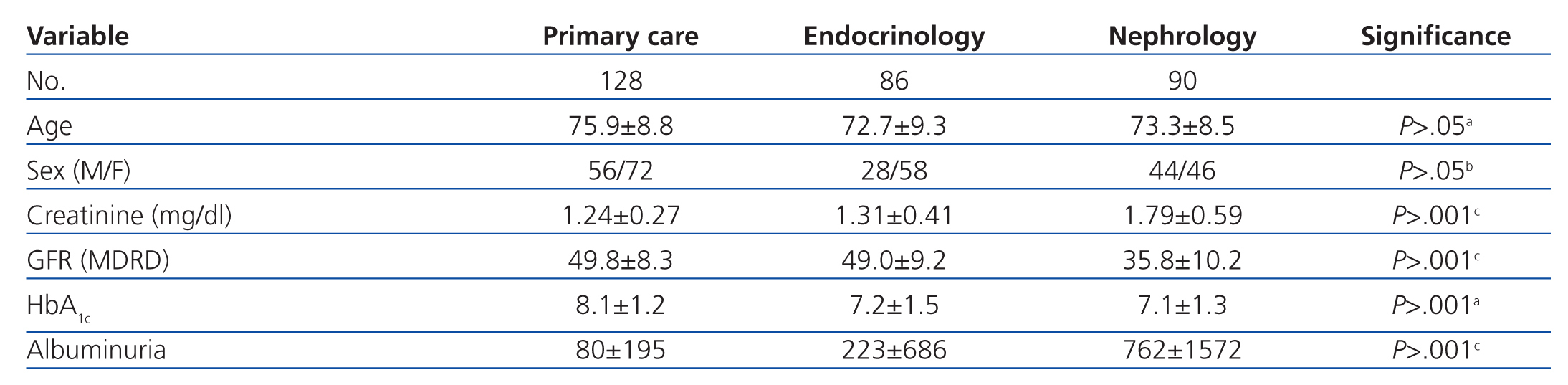
Patients were from primary care (128), outpatient endocrinology and nutrition (86), and outpatient nephrology units (90). The characteristics of each group are summarised in Table 1. Patients derived from primary care were on average older, the endocrinology group had a higher proportion of female patients, and those from nephrology had higher creatinine and proteinuria rates and a lower GFR.
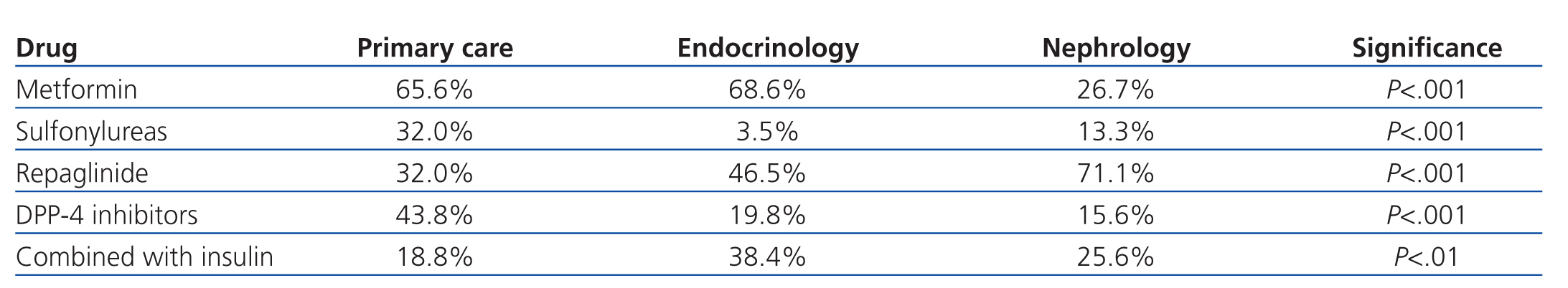
The most commonly used ADO was metformin (167 patients, 54.9%) followed by repaglinide (145 patients, 47.7%). DPP-4 inhibitors were prescribed in 87 patients (28.6%), and 56 (18.4%) received sulfonylureas, with glimepiride being the most commonly prescribed. The ADO prescribed was associated with insulin in 80 patients (26.3%). Statistically significant differences were observed in the prescription of ADOs between the three groups: metformin was used less frequently and repaglinide was used to a greater extent in patients derived from nephrology, DPP-4 inhibitors were used more frequently in primary care, and 70% of these cases involved metformin. Endocrinology patients were most commonly prescribed ADO with insulin, and were less frequently prescribed sulfonylureas (Table 2).
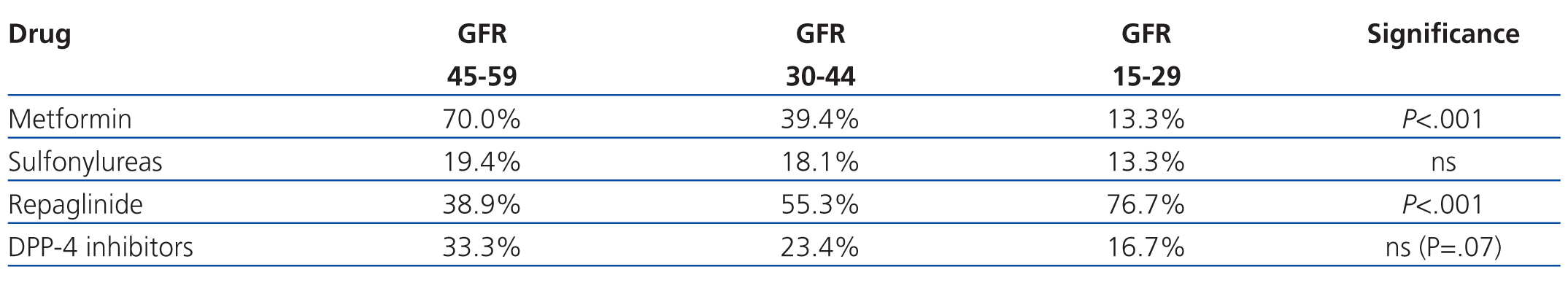
As regards the use of the different types of ADO according to severity of GFR, metformin was the least commonly used drug with a GFR of 15-29ml/min/1.73m2 (4/30 patients, 13.3%), along with sulfonylureas, but it was the most commonly prescribed drug in patients with a GFR of 45-59ml/min/1.73m2 (126/180 patients, 70.0%) (P<.001). The opposite occurred in the case of repaglinide, as this was the most commonly prescribed drug in patients with a GFR of 15-29ml/min/1.73m2 (23/30 patients, 76.7%), but was less commonly prescribed at 45-59ml/min/1.73m2 levels (70/180 patients, 38.9%) (P<.001). DDP-4 inhibitors were less frequently prescribed at lower GFR values, although this difference did not reach statistical significance (P=.07), and GFR had no apparent correlation with the use of sulfonylureas (Table 3).
DISCUSSION
In recent years, several different consensus documents1-3 and guidelines4,5 have been published that coincide on the recommendation to use metformin as the glucose-lowering drug of choice for patients with type 2 DM. However, no such agreement exists as regards the level of renal damage from which the use of this drug is contraindicated due to the potential risk of lactic acidosis (LA), a rare but severe complication that can arise.1-5
With the objective of evaluating and comparing medical practice among different groups of health care professionals (primary care, endocrinology, and nephrology departments) in our health area, and in light of the information provided in the available consensus documents and guidelines, we have reviewed the characteristics of treatment with ADO in a group of 304 patients diagnosed with type 2 DM and a GFR (MDRD-4) <60ml/min/1.73m2, focusing primarily on the use of metformin. Overall, metformin was the most commonly used ADO, followed by repaglinide, DPP-4 inhibitors, and sulfonylureas (mainly glimepiride).
Upon analysis of the data by department, we observed that the most commonly used drug was metformin both in primary care and endocrinology units, with a significantly lower rate of use by nephrologists, which also occurred with sulfonylureas. We also observed that sulfonylureas and DPP-4 inhibitors were more commonly used in primary care, repaglinide was more commonly used in nephrology units, and repaglinide in combination with insulin in endocrinology departments. The more intensive use of DPP-4 inhibitors in primary care was associated with the simultaneous use of metformin (70%), with appearance of these associations (vildagliptin/sitagliptin with metformin) occurring in recent years. The lower rate of use of metformin in nephrology units can be explained by the higher mean plasma creatinine level (1.79±0.59mg/dl) and significantly lower GFR (10.2±35.8ml/min/1.73m2) as measured by MDRD-4 than in the other groups. Furthermore, sulfonylureas were used at a lower rate and repaglinide was used more frequently by nephrologist, as repaglinide has a short half-life and can be used in patients in advanced stages of renal failure.1
Upon analysis of the use of metformin and its correlation with GFR (MDRD-4), we found that the majority of patients (70%) had a GFR of 45-60ml/min/1.73m2, 39.4% had a GFR of 30-44ml/min/1.73m2, and only 13.3% had a GFR of 15-29ml/min/1.73m2. As such, no patient fell within the recommendations made by the SED and AACE/ACE, which contraindicate the use of metformin at GFR<60ml/min,1,2 although it is in line with the recommendations from Canadian and Australian clinical guidelines4,5 and with the non-explicit recommendations from other consensus documents.3 In any case, most doctors consider a cut-off point of 30ml/min to be an absolute contraindication for the use of metformin.
Metformin and sulfonylureas were prescribed in 13.3% of patients with a GFR<30ml/min/1.73m2, even though the use of these drugs in patients with such a low GFR is contraindicated and does not fall within the ranges observed in clinical recommendations. This trend is reported in other studies as well, in which as many as 27% of patients that received metformin had some contraindication for its use.12-14 In these studies, no mention is made to the reasons justifying the use of metformin in patients with contraindications for the drug, although doubts are raised as to the maintenance of this therapy in many patients (41%-75%), despite these contraindications.12-14 The four patients that received metformin with a GFR<30ml/min/1.73m2 were 69-95 years old and had a plasma creatinine level of 1.9-2.2mg/dl (GFR: 23-28ml/min/1.73m2).
The basis for different levels of metformin prescribed according to GFR lies in the possible increase of risk for LA in patients with renal failure, since lactic acid is eliminated through filtration and active tubular secretion. The association between LA and renal failure in patients with type 2 DM is currently under debate, to say the least. In a review of the Cochrane database,15 no cases of fatal or non-fatal LA were observed when combining the information for 206 comparative trials performed with a total of 47 846 patients/year treated with metformin and 38 221 patients/year treated without metformin. In a systematic literature review, again no differences were observed when analysing the incidence of LA between patients treated with and without metformin, although in this study, the mean incidence of LA was 8.4 cases/patient/year in the group with metformin, and 9 cases/patient/year in the other,16 which is higher than the rates reported elsewhere (3.3 cases/patient/year in groups treated with metformin vs 4.8 cases/patient/year in groups treated with other sulfonylureas).17 These studies concluded that there is no increased risk of LA, and that the primary cause for this condition is systemic dysfunction.
Although no randomised studies have been carried out regarding the use of metformin in renal failure, some have reviewed the data from its use in patients with varying stages of renal damage, and it has been generally established that, based on the minimal existence of complications and the potential benefits of the drug, it can generally be used with caution in patients with a GFR of 30-60ml/min.13-19 Recently, recommendations have been published that support the use of metformin in patients with a GFR of 45-60ml/min, with control tests for renal function every 6 months ; and in patients with a GFR of 30-45ml/min, a reduction of the dose by half and renal function tests every 3 months would be necessary. However, they maintained the absolute contraindication for prescribing metformin when GFR<30ml/min.20
In the last 15 years, we have diagnosed only 2 patients with LA, and in neither case had metformin been prescribed when GFR<60ml/min/1.73m2. Both cases were triggered by dehydration from severe gastroenteritis and prerenal acute renal failure.
Upon reviewing the technical data sheet for metformin, which is the legal document regulating its use, we found that explicit contraindications are stated for its use in patients with renal failure or renal dysfunction (creatinine clearance <60ml/min), although no reference is made to adjusting the measure to body surface area.9 In light of the technical data sheet and guideline recommendations and the analysis of our results, we should consider whether our medical conduct is correct and within the legal framework. We would like to reflect on the information provided in the metformin technical data sheet. Is creatinine clearance, as described in the drug’s data sheet, the currently used standard method for measuring renal function?
Currently, the nephrological scientific community does not consider creatinine clearance to be the most adequate parameter for measuring GFR. In 2002, the National Kidney Foundation (NFK) – Kidney Disease Outcomes Quality Initiative (KDOQI) published a guideline for the evaluation, classification, and stratification of chronic kidney disease, and recommended estimating GFR, the currently used method in clinical practice, to evaluate the level of renal dysfunction and its progression through time, using formulas that take into account serum creatinine, such as Cockcroft-Gault (CG) and MDRD.21 Spanish Society of Nephrology guidelines also recommend the use of CG and MDRD for calculating GFR (level B evidence).22
Although the comparison between CG and MDRD is under debate, primarily based on the characteristics of the population studied and the method used for calculating serum creatinine,23 which results in underestimation of GFR>60ml/min/1.73m2,24 the majority of authors and scientific associations have used the MDRD-410 formula as the reference method due to its ease of application in clinical laboratories and the fact that patient weight is not needed.25 The laboratory at our hospital uses the MDRD-4 formula for calculating GFR. Upon analysis of the population characteristics from our study, we observed that all of our patients were diabetics, with a mean age of 74.2 years, and 59.2% had a GFR of 45-59ml/min/1.73m2, meaning that they were far from the characteristics of the population of the MDRD study, in which only 6% of patients were diabetics, with a mean age of 51 years, and chronic renal failure at a mean GFR of 40ml/min/1.73m2.26
Some guidelines also establish the possibility of using serum creatinine as the reference method for prescribing ADO (1.5mg/dl for men and 1.4mg/dl for women).2 Creatinine is not currently considered a good parameter for measuring renal function (KDOQI); in addition, the percentages of patients included in each stage of renal failure would vary considerably when compared to using MDRD as the reference method, decreasing the potential number of patients that could use metformin.27
The search for a new equation that would facilitate a better and more accurate GFR in different populations and patients with a GFR>60ml/min/1.73m2 led the U.S. National Institute of Diabetes and Digestive and Kidney Diseases to develop a new equation in 2009 (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]). This formula uses the same variables as the MDRD-4 and allows for a better estimate of GFR in patients with a GFR>60ml/min/1.73m2, which decreases the rate of false positives and improves drug prescription and the use of contrast dyes.28
Later studies in different study populations appear to confirm these data.29,30 The recommendations for using metformin in patients with a GFR of 30-60ml/min/1.73m2, as expressed in consensus documents, guidelines, studies, and medical practice, suggest the possibility of modifying the technical data sheet for metformin, not only in terms of contraindications for its use in patients with renal failure, but also regarding the use of creatinine clearance as a parameter for measuring renal function. We understand that this is a complex and costly process in which scientific associations should play a greater role. New evidence, studies, experience, and better understanding are necessary to change the recommendations and contraindications established for a drug. We should also conduct studies in patients with renal failure, who are normally excluded from clinical trials, in order to fully understand this issue.
We conclude that metformin, a drug recommended by various consensus documents and guidelines for the treatment of patients with DM, is a safe, useful, and cheap ADO. However, its current technical data sheet, a document establishing legal constraints, contraindicates its use in patients with creatinine clearance <60ml/min. Although no randomised studies have been performed in populations with renal failure, meta-analyses and retrospective and observational studies suggest that metformin can be used with caution, instructing the patient, and reducing the dosage in patients with a GFR of 30-60ml/min/1.73m2.
Currently, creatinine clearance has been replaced by MDRD as the method of choice for estimating GFR, although it underestimates GFR in patients with GFR>60ml/min/1.73m2 and is not validated for all populations, including many of the patients that we see on a regular basis. The nephrological community should develop formulas for estimating GFR with greater accuracy in all types of patients, as well as standardise the technical data sheets for drugs in terms of reference parameters used for measuring renal function. We believe that scientific associations, the ministry of health, and pharmaceutical laboratories should review the potential modification of the technical data sheet for metformin, with the goal of allowing health professionals to work within the legal framework established for this drug.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Characteristics and differences between the three patient groups according to origin
Table 2. Differences in the use of oral antidiabetic drugs by health care department
Table 3. Differences in the use of oral antidiabetics according to glomerular filtration rate