Introducción: Los valores de los compartimentos corporales proporcionados por los dos sistemas de bioimpedancia más utilizados en España (bioimpedancia de monofrecuencia vectorial [BIVA] y bioimpedancia multifrecuencia espectroscópica [BIS]) son diferentes y no pueden intercambiarse. Objetivo: Analizar si la variabilidad intermétodo es debida a la diferente lectura de las variables bioeléctricas realizadas por los monitores o a las ecuaciones utilizadas por cada uno de ellos para el cálculo de los volúmenes y masas corporales. Otro objetivo fue comprobar si, a pesar de la variabilidad intermétodo, la clasificación de los estados de hidratación definidos por ambos monitores es concordante. Material y métodos: Estudio de corte transversal. En 54 enfermos tratados con hemodiálisis se hizo un análisis de bioimpedancia con los monitores BIVA y BIS inmediatamente antes de una sesión de diálisis. En 38 de ellos se repitió el estudio con el monitor BIVA al finalizar la misma sesión de diálisis. Resultados: Los datos de resistencia y ángulo de fase proporcionados por el monitor BIVA y por el monitor BIS a la frecuencia de 50 kHz son concordantes. En el caso de la resistencia, la variabilidad es de 1,3%, y el coeficiente de correlación intraclase, de 0,99. Para el ángulo de fase, la variabilidad es del 11,5%, y el coeficiente de correlación intraclase, de 0,92. Los valores del volumen de agua corporal total, agua extracelular, masa grasa y masa celular tienen un sesgo y una variabilidad no admisibles en la práctica clínica y el coeficiente de correlación intraclase indica que la concordancia es mediocre. En el sistema BIVA se define hiperhidratación o deshidratación según el vector estuviera en el eje de hidratación por debajo o por encima de la elipse de tolerancia de 75%, tanto pre como posdiálisis. El sistema BIS utiliza dos criterios de hiperhidratación prediálisis: OH (exceso de hidratación prediálisis) superior a 2,5 litros o mayor del 15% del volumen de agua extracelular. El grado de equivalencia con los resultados del monitor BIVA fue mejor con el segundo criterio (índice kappa 0,81, concordancia excelente), que con el primero (índice kappa 0,71, concordancia aceptable). El sistema BIS define la normohidratación posdiálisis cuando la diferencia entre OH y volumen ultrafiltrado está comprendida entre –1,1 y 1,1 litros, y su concordancia con el BIVA fue aceptable (índice kappa ponderado 0,64). Conclusiones: Los monitores BIVA y BIS utilizados proporcionan lecturas similares de los parámetros bioeléctricos y la gran variabilidad observada en la cuantificación de volúmenes y masas corporales debe ser atribuida a las diferentes ecuaciones utilizadas para su cálculo. Sin embargo, los criterios utilizados por ambos sistemas para definir los estados de hidratación pre y posdiálisis tienen una equivalencia aceptable.
Introduction: The values of body composition provided by the two most commonly used bioelectrical impedance systems in Spain, single-frequency bioelectrical impedance vector analysis (SF-BIVA) and multi-frequency bioelectrical impedance spectroscopy (MF-BIS) are different and not comparable. Objective: Analyse whether the inter-method variability is due to bioelectrical variables measured by the different monitors, or rather due to the equations used to calculate body volume and mass. Another objective was to determine whether, despite the inter-method variability, the classification of hydration status by the two methods is consistent. Material and Methods: Bioelectrical impedance was measured by SF-BIVA and MF-BIS immediately before a dialysis session in 54 patients on haemodialysis. In 38 patients, the study was repeated by SF-BIVA at the end of the same dialysis session. Results: Resistance and phase angle values provided by the two monitors at a frequency of 50kHz were consistent. For resistance, variability was 1.3% and the intra-class correlation coefficient was 0.99. For phase angle, variability and the intra-class correlation coefficient were 11.5% and 0.92, respectively. The volume values for total body water, extracellular water, fat mass and body cell mass were biased, with a level of variability that would not be acceptable in clinical practice. The intra-class correlation coefficient also suggested a poor level of agreement. SF-BIVA systems define overhydration or dehydration as a vector below or above the tolerance ellipse of 75% on the longitudinal axis. MF-BIS uses two criteria for pre-dialysis hyper-hydration: overhydration (OH) greater than 2.5 litres, or greater than 15% of extracellular water. The degree of equivalence with the results of the SF-BIVA monitor was better with the second criterion (kappa: 0.81, excellent agreement) than with the first one (kappa: 0.71, acceptable agreement). The MF-BIS system defines post-dialysis normal hydration as a difference between OH and ultrafiltratation volume between –1.1 and 1.1 litres and agreement with the SF-BIVA system for this parameter was acceptable (weighted kappa index: 0.64). Conclusions: The MF-BIS and SF-BIVA systems provide similar readings for bioelectrical parameters, and the wide variation in the quantification of volume and body mass must be attributed to the different equations used for calculation. Furthermore, the criteria used by both systems to define both pre- and post-dialysis hydration have an acceptable level of equivalence.
INTRODUCTION
Bioelectrical impedance analysis allows us to quantify the different human body compartments and provides useful information for evaluating nutrition and hydration status. Simple, easy to use monitors with an accessible price range have led to the current widespread use of this technology in nephrology departments. This is evidenced by the large number of reports regarding bioelectrical impedance that were presented at the last three national conferences in this medical specialty.
Bioelectrical impedance monitors obtain electrical parameters from the human body (resistance, reactance, and phase angle), and calculate body mass and volume using predictive equations that take into account electrical data and other variables, such as weight, height, age, and sex. These equations vary between the different types of monitors. The majority only takes into account resistance, and on many occasions they are difficult to learn.1-6
Multi-frequency bioelectrical impedance spectroscopy (MF-BIS) and single-frequency bioelectrical impedance vector analysis (SF-BIVA) are the two most commonly used bioelectrical impedance systems in Spain.7,8 Comparative studies reported that the two systems provide different results for the body compartments and methods are not interchangeable due to the high inter-method variability.6,9-12
The aim of our study was to determine whether inter-method variability is due to differences in the way monitors read bioelectrical variables, or due to the equations used by each system to calculate body mass and volume. Another objective was to test whether, despite the inter-method variability, the classification of a patient’s hydration status was consistent across the two different systems. This study was performed in patients with stage 5 chronic kidney disease being treated with haemodialysis.
MATERIAL AND METHODS
Ours was a cross-sectional study of 54 patients on periodic haemodialysis who underwent bioelectrical impedance analysis using both MF-BIS and SF-BIVA. The mean patient age was 69±14 years (range: 34-92 years); 36 patients were male and 18 were female. All patients were clinically stable, with no signs or symptoms of heart failure. Mean body mass index was 26.5±3.9 (range: 18.3-38.3; confidence interval: 25.5-27.6). The bioelectrical impedance analysis was performed before the haemodialysis session, with the patient lying in a supine position, placing electrodes at the wrist and ankle of the side of the body free from vascular accesses, following standard protocol. We first took measurements with the SF-BIVA monitor, which used a 50kHz frequency (ElectroFluidGraph [EFG] analyser, Akern SRL, Florence, Italy), and then with the MF-BIS device (BCM monitor, Fresenius Medical Care, Bad Homburg, Germany), which took readings at 50 frequencies with a range of 5kHz-1000kHz. In 38 patients, we repeated the bioelectrical impedance analysis using SF-BIVA after completing the haemodialysis treatment. We used the same electrodes, which were left in place throughout the session.
The SF-BIVA monitor provides values for resistance, reactance, and phase angle at a 50kHz frequency. The MF-BIS monitor provides values for resistance and phase angle for each frequency used. In order to compare the bioelectrical data from the two monitors, we used the results from phase angle and resistance produced by the MF-BIS system at 50kHz. In order to analyse hydration status, we assigned a score (ordinal scale) from 1 to 7 to the results produced by the SF-BIVA monitor (from -3 to +3) along the major axis of the three tolerance ellipses (95%, 75%, and 50%) from the lower pole of greater hydration to the upper pole of less hydration, as proposed by Piccoli.1 With the MF-BIS monitor, hydration status was determined using the pre-dialysis hyper-hydration value (overhydration: OH) provided by the monitor. Post-dialysis OH was calculated by subtracting the ultrafiltration volume from the OH value (post-HD OH).
Hydration status was defined using the following criteria. With the MF-BIS monitor, we used two criteria to define pre-dialysis overhydration: an OH volume greater than 15% the extracellular water volume (ECW)13 and a total OH volume greater than 2.5 litres.14 Post-dialysis hydration status was considered normal when post-HD OH was between -1.1 litres and 1.1 litres; overhydration: OH>1.1 litres, and dehydration: OH<-1.1 litres.15 When using the SF-BIVA monitor, hydration was considered normal when the impedance vector on the hydration axis was within the 75% tolerance ellipse in pre- and post-dialysis measurements.16 Under this criteria, we defined pre-dialysis overhydration as an impedance vector in the pre-dialysis measurement below the 75% tolerance ellipse (on the ordinal hydration scale, this corresponds to values +3 and +2). In the post-dialysis assessment, the same criteria were used to define overhydration; normal hydration in the post-dialysis period was defined as a post-dialysis impedance vector within the 75% tolerance ellipse (values +1, 0, and -1 on the ordinal scale) and dehydration was defined as a post-dialysis impedance vector above the 75% tolerance ellipse (corresponding to values -2 and -3 on the ordinal scale).17
For our statistical analysis, we presented results in terms of mean ± standard deviation. Our data had a normal distribution (Kolmogorov-Smirnov test), and so we only used parametric tests. The difference between SF-BIVA and MF-BIS values for each parameter was defined as the bias between the two systems. This same difference in absolute values expressed as a percentage of the arithmetic mean for both values (relative difference) allowed us to examine the variability between the two measurement methods. The correlation between the two different methods was measured using Pearson’s coefficients. For quantitative variables, we performed an intra-class correlation analysis,18 which varied between 0 (no agreement) and 1 (total agreement). For binary and ordinal variables, we used kappa index and weighted kappa index tests.19 For the kappa index, a level of agreement >0.40 was considered acceptable, and excellent at values >0.75. We compared means using Student’s t-tests and ANOVA, as necessary. A P-value <.05 was considered statistically significant.
RESULTS
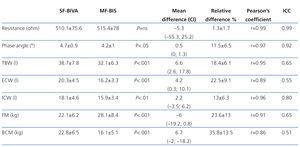
Table 1 shows resistance and phase angle values produced by SF-BIVA and MF-BIS monitors at a frequency of 50kHz, and the body composition values obtained using the two methods. The values for resistance have a minimal variability, and the intra-class correlation coefficient suggests that the inter-method agreement is almost absolute. However, the values for phase angle were statistically different. Even so, the level of variability is acceptable from a clinical standpoint (11.5%), and the intra-class correlation coefficient of 0.92 indicates excellent inter-method agreement.
Of the different body composition variables measured by the two systems, only intracellular water volume (ICW) had an acceptable level of variability between the values for the two different types of monitors (13%); in all other variables, bias and variability are very high. Although the Pearson’s correlation coefficient suggests that there is a good correlation between the values obtained by the two systems, the intra-class correlation coefficient indicates that the level of agreement is mediocre.
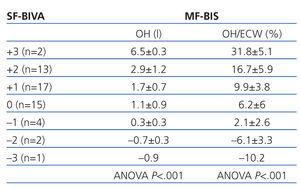
Table 2 displays the MF-BIS parameters associated with hydration status (OH and the OH/ECW ratio) for the 7 different levels of the ordinal scale that measure hydration in the SF-BIVA monitor, showing good correlation between the two different methods.
The classification of patients according to pre- and post-dialysis hydration status is expressed in Table 3 and Table 4. In pre-dialysis patients, the kappa index for diagnosing overhydration was 0.81 if the diagnostic criterion of overhydration with the MF-BIS system was an OH/ECW>0.15 (excellent agreement) and 0.71 if the diagnostic criterion was an OH>2.5 (acceptable agreement). In post-dialysis measurements, the mean weighted kappa index was 0.64 (acceptable agreement).
DISCUSSION
The different manufacturers of bioelectrical impedance monitors assure us that the procedures they use to calculate body mass and volume are validated against reference methods, both in healthy subjects and patients suffering a wide range of pathologies, but the results obtained with the different bioelectrical impedance systems demonstrate a substantial inter-method variability.6,9-12 The aim of our study was to determine whether this inter-method variability was due to different results obtained in the measurement of bioelectrical parameters, or due to the equations used by each system to quantify body compartments.
The measurements of resistance and phase angle provided by SF-BIVA and MF-BIS monitors at a frequency of 50kHz have a high level of agreement. The mean variability for resistance was only 1.3%, similar to the intra-individual variability rate.3,16 The intra-class correlation coefficient (0.99) suggests that the agreement between the two systems is virtually absolute. The measurements of phase angle were different from a statistical point of view, but the mean variability (11.5%) could be negligible from a clinical standpoint, and the intra-class correlation coefficient (0.92) indicates a high level of agreement. We can conclude that the two monitor systems make very similar measurements of bioelectrical parameters at a frequency of 50kHz.
The measurements for total body volume, extracellular water, intracellular water, fat mass, and body cell mass, showed high variability and bias. As in other studies performed using these same monitors,10,11 we observed that the SF-BIVA system yields higher values than the MF-BIS system for all compartments analysed, except for fat mass. The best correlation between the two systems occurred in ICW (mean bias: 2.2 litres; mean variability: 13%; intra-class correlation coefficient: 0.80), which is acceptable. For all other compartments, the bias and variability were not negligible, and the intra-class correlation coefficient indicates that only a mediocre equivalence exists between the two systems. The Pearson’s coefficient demonstrated a close correlation between the results produced by the two systems, but this is not a valid test for a concordance analysis.18-20 The majority of equations that determine body volume and mass only use resistance as the bioelectrical parameter.1,3,5 Since the level of agreement in the readout for resistance at a frequency of 50kHz is virtually absolute, we must assume that the inter-method variability observed is attributable to the different bioelectrical models and equations used by each bioelectrical impedance device.
In addition to quantifying body mass and volume, the different bioelectrical impedance systems utilise certain criteria to classify patients based on hydration status. For pre-dialysis values, the MF-BIS system uses the parameter of OH, expressed in litres,14 or in a percentage of ECW13;and for post-dialysis values, the estimated post-dialysis OH in litres.15 The SF-BIVA system defines pre- and post-dialysis hydration status by applying an ordinal scale to tolerance elipses.1,17 Upon analysing the equivalence of the two systems for classifying patients according to hydration status, we observed that the level of agreement was good both for defining pre-dialysis overhydration status and post-dialysis normal, over-, and dehydration. Although the results for the different body water compartments obtained from the two systems are not interchangeable, the criteria used to define hydration status had a very high level of correlation in classifying patients.
Phase angle is a bioelectrical parameter associated with nutrition, and has a prognostic value in patients with renal failure.21-24 When evaluating this parameter, we must keep in mind that phase angle varies with hydration status,25,26 and increases following haemodialysis sessions.16,27 Our study suggests that in the pre-dialysis period, the two monitor systems have an acceptable level of agreement, and that phase angle obtained by either device can have the same significance when analysing patient prognosis or nutrition status.
We conclude that the SF-BIVA and MF-BIS monitor systems produce comparable results for resistance and phase angle at a frequency of 50kHz. The measurement of body compartments does have a high inter-method variability that is probably due to the equations used. However, the different criteria used for defining hydration status by each device are comparable and classify patients quite consistently.
The choice of which bioelectrical impedance system to use in patients on dialysis has caused considerable controversy. With our results, we cannot conclusively say which one is more advisable. If phase angle and ICW are used as nutritional parameters and hydration status is measured following our method, the results from the two different systems are comparable, and in our opinion, both procedures are clinically useful.
Acknowledgements
We would like to thank Andrés Sánchez Iglesias, physics professor, for assistance in comprehending the functioning of bioelectrical impedance analysis.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Resistance and phase angle values
Table 2. Relationship between hydration status according to the SF-BIVA monitor and overhydration according to the MF-BIS monitor
Table 3. Pre-dialysis hydration status. Concordance between the definition criteria used by SF-BIVA and MF-BIS systems
Table 4. Post-dialysis hydration status. Concordance between the definition criteria used by SF-BIVA and MF-BIS systems