El trasplante renal es la mejor opción de tratamiento de la insuficiencia renal crónica y la escasez de donantes de cadáver ha ocasionado la potenciación de los programas de donante vivo. Dado que una proporción no desdeñable de las parejas donante-receptor son incompatibles entre sí, ya sea por incompatibilidad de grupo sanguíneo o por prueba cruzada positiva, uno de los retos más importantes de la última década ha sido la solución de dicho problema. Para ello se han iniciado los programas de trasplante cruzado o intercambio de donantes en sus distintas combinaciones y se han consolidado con unos excelentes resultados el trasplante ABO incompatible y el trasplante con prueba cruzada previa positiva. Para eliminar los títulos de anticuerpos anti-HLA y las isoaglutininas disponemos de diferentes recursos, entre los que cabe destacar la plasmaféresis, la inmunoadsorción, la infusión de inmunoglobulinas, el uso de Rituximab® y la esplenectomía. Todos ellos requieren del uso concomitante de una inmunosupresión potente y de una adecuada profilaxis antiinfecciosa. Los resultados obtenidos con los donantes incompatibles son hoy en día excelentes y totalmente equiparables a los obtenidos con el trasplante de donante vivo compatible.
Renal transplant is the best option of treatment of chronic kidney disease and the shortage of cadaveric donors has caused the rapid increase of living donor programs. Provided that an important proportion of the donor-recipient pairs are incompatible between them, ABO incompatibility or positive cross-match test, one of the most important challenges of last decade, has been the solution of the above mentioned problem. For it there have begun the crossed-over transplant programs (also called donors' exchange programs) in his different combinations and these kind of transplants has been consolidated by an excellent results. To eliminate the titles of anti-HLA antibodies and the isoaglutinines we have different resources, beeing the most importants plasmapheresis, the immunoadsortion, immunoglobulin infusion, Rituximab use and splenectomy. They need all of them of the concomitant use of a powerful immunosuppression and of a suitable antiinfectious prevention. The results obtained with the incompatible donors are nowadays excellent and totally comparable to the obtained ones using living compatible donors.
¿
INCOMPATIBLE LIVING DONORS
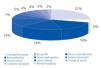
The lack of cadaveric donors, especially for younger age groups, has promoted the rapid and progressive increase in living donations in Spain. For this reason, the Organización Nacional de Trasplantes (Spanish National Transplant Organisation, or ONT), regional organisations and individual centres have invested openly in promoting living donations. However, there is a significant number of potential living-donor transplants where incompatibility is detected between donor and recipient. The main causes are blood group incompatibility and a positive crossmatch due to preformed antibodies against HLA antigens. If the titres of antibodies against the ABO system or the HLA are not reduced to zero, the recipient will experience severe acute rejection provoked by the antibodies, which will cause very early graft loss. Until recently, these living-donor transplants were rejected. The Fundación Puigvert (Puigvert Foundation) rejected 100 potential living donors for the period 2000-2008, attributing 28% of these rejections to donor-recipient incompatibility (Figure 1). Fortunately, there are two options that can now be offered to bring about the transplant:
1. For ABO incompatibility, there is the crossover transplant or the ABO incompatible transplant.
2. For positive crossmatch, there is the crossover transplant or desensitisation prior to kidney transplantation.
ABO INCOMPATIBILITY
Everyone has naturally-occurring isoagglutinins against those ABO antigens they do not have. Those antigens are in erythrocytes, lymphocytes, platelets and endothelial and epithelial cells and they are the basis of various blood groups. Thus, a type O individual will have preformed isoagglutinins against types A and B; a type A individual will have them against type B; a type B individual will have them against type A and a type AB individual will not have them against any type. There are also two subtypes for type A: A1 and A2. The majority of the population has type A1, and type A2 is qualitatively and quantitatively much less common, so the risk of acute rejection caused by antibodies in the case of ABO incompatibility is lower for type A2 donors. The highest isoagglutinin titres are detected in type O recipients.1
ABO-INCOMPATIBLE DONATIONS
Organ transplantation with ABO blood group incompatibility has been carried out for more than 40 years, however, the final push towards ABO-incompatible transplantation came from Japanese groups at the end of the 90s. They published their initial series, showing good results and a clear improvement in these results in the last 5 years.2-4 Currently, this procedure has had extensive implementation in Japan and is steadily expanding both in the U.S. and Europe.5,6
ABO-incompatible transplantation is possible as long as the isoagglutinin titre against the donor is very low. Since these are natural antibodies, most subjects have more or less elevated titres that are rarely low enough to proceed to transplantation immediately. In order to perform a transplant, the concentration of isoagglutinins needs to be reduced to certain levels that significantly reduce the occurrence and severity of antibody-mediated acute rejection. At the same time, the immediate re-synthesis of these isoagglutinins must be prevented. Concomitant immunosuppression must prevent antibody-mediated rejection.
REDUCING ISOAGGLUTININ TITRES
Various methods have been used to reduce isoagglutinin titres:
1. Splenectomy has usually been used in most desensitisation protocols for ABO-incompatible transplantation with good results, especially in Japanese groups.7 However, the introduction of rituximab has made splenectomy a non-essential procedure. It is therefore being withdrawn from desensitisation protocols, avoiding its associated morbidity and mortality.8
2. Plasmapheresis and immunoglobulin infusion have been widely used by Japanese groups with good results. The results for both techniques depend on the reduction of isoagglutinin titres. Transplantation is not performed until the titre is lower than 1:8 or 1:16, depending on the transplant groups. Usually between 4 and 5 sessions are conducted prior to transplant and between 3 and 5 sessions after transplant. Immunoglobulin infusion is usually 100mg/kg after each plasmapheresis session.9
3. Antigen-Specific Immunoadsorption (ASI), which uses polycarbonate columns filled with Sepharose with blood group A and B trisaccharides linked to its surface (Glycosorb®). The removal of isoagglutinins using this technique avoids plasmapheresis. The number of immunoadsorption sessions will depend on the previous isoagglutinin titres. As with plasmapheresis, pretransplant titres must be lower than 1:8.6
4. Rituximab is a humanised murine monoclonal antibody that binds to CD20, which is expressed in most B cells. Its inclusion in desensitisation protocols has brought about the near eradication of splenectomy and has improved results in recent years. Since most plasma cells do not express CD20, the use of rituximab must be combined with plasmapheresis or immunoadsorption to eliminate isoagglutinins.10-12 Usually, patients receive one or two doses of 375mg/m2 of rituximab. European protocols based on ASI and rituximab state that treatment should begin between one and four weeks before the estimated transplant date. ASI sessions should be repeated as many times as necessary prior to transplantation to reach isoagglutinin titres (IgG and IgM) below 1:8. Isoagglutinin levels should be measured during the three weeks following transplantation and ASI should be performed if necessary. Usually four sessions are necessary prior to transplantation and another three after transplantation, although there is considerable variability among individuals.
If isoagglutinin titres are very high, desensitisation is likely to be ineffective, whether it is performed with plasmapheresis or ASI. As a guideline, titres above 1:256 or 1:512 predict poor response to treatment and it is therefore advisable to rule out ABO-incompatible transplantation.
5. Powerful immunosuppression. The process of immunomodulation is completed with the administration of an immunosuppression regimen that includes tacrolimus, mycophenolic acid, corticosteroids and polyclonal or monoclonal anti-lymphocyte antibodies.7
LONG-TERM RESULTS OF ABO-INCOMPATIBLE TRANSPLANTATION
According to Japanese groups, these results are almost the same as those for ABO-compatible transplantation.3,13 Takahashi et al reported the data from an historical cohort of 441 ABO-incompatible transplant recipients with patient survival rates at 1, 3, 5, 7 and 9 years of 93%, 89%, 87%, 85% and 84%, respectively. Graft survival for the same periods were 84% 80%, 71%, 65% and 59%, respectively. These are excellent results and are the same as those for ABO-compatible donors for the same time periods.4
Antibody-mediated acute rejection may appear in the first weeks after transplantation. After 2 or 3 weeks, many patients have spontaneous increases in titres without suffering humoral rejection. This accommodation-like phenomenon may be clearly confirmed when C4d is detected in protocol biopsies in the absence of acute rejection.14,15
Crossover transplant
Crossover living-donor kidney transplantation consists of reciprocally and simultaneously exchanging kidney donors between different donor-recipient pairs who cannot donate directly to their recipients for immunological or other reasons. The most common reasons are ABO-incompatibility and positive crossmatches. There are different types of crossover kidney transplantation exchange:
1. Two-way exchange: The donor in pair A gives a kidney to recipient in pair B and the donor in pair B gives a kidney to recipient in pair A.
2. Three/+-way exchange: In order to do this, a state organisation is needed that has a database with numerous donor-recipient pairs who want a crossover transplant. The donor of one pair gives a kidney to a recipient in the next and so on until the donor of the last interchangeable pair is used for the recipient of the first donor who started the chain.
3. Exchanges with the cadaveric-donor waiting list. A donor-recipient pair who is incompatible with each other gives the donor kidney to a recipient on a cadaveric-donor waiting list and receives top priority on that list in exchange. The disadvantage is that although it often benefits recipients of group A cadaveric organs, it is detrimental to recipients of group O cadaveric organs.
4. Age difference exchange. A donor-recipient pair who are compatible with each other but have a significant age difference (the donor being older), decide to exchange with a donor-recipient pair who are younger but incompatible with each other.
5. Exchange chains that start with an altruistic donor and end with a recipient on a cadaveric-donor waiting list. Chains that start with an altruistic donor also consider, on occasion, the displacement of the kidney instead of the donor, the commitment of the donor to give up their kidney in the future when their recipient has already been transplanted, the use of ABO-incompatible exchanges to avoid a positive crossmatch, accepting friends as donors, etc.16
The crossover transplant programme is an alternative to direct incompatible transplants because it facilitates finding a donor-recipient combination that avoids the need for intense desensitisation and more potent immunosuppression than usual. However, there are no reports of differences in survival between both types of transplant.13,17 Crossover transplantation also avoids the high costs of desensitisation and immunoadsorption that are carried out in cases of ABO-incompatible or initially positive crossmatch transplantation.
There are various ethical and psychological issues that also need to be kept in mind when considering both transplant possibilities in a specific donor-recipient pair:
1. Against crossover transplant:
a) The donor may only be willing to give their organ to their recipient.
b) The donor may be indirectly coerced.
c) The failure of a recipient may dramatically affect the recipient and their family member who donated to another recipient.
d) The recipient may view their crossover donor to be of "worse quality" than their original donor.
2. Against ABO-incompatible transplant:
a) The financial cost to the system
b) Possible excessive immunosuppression
c) A donor-recipient pair undergoes transplantation and it does not benefit a third party.
Table 1 shows the main advantages and disadvantages of each type of transplant.
When deciding which option to offer incompatible patients we must consider the real probability of transplantation in case of choosing crossover transplantation. For those cases where there are blood group incompatibilities, if the donor and recipient are either type A or B, the possibility for crossover is very high. However, the possibility of crossover is much lower for type O recipients unless the chance arises for an exchange with a donor-recipient pair who wishes to improve the age of the donor. In case of a positive crossmatch, the possibility of exchange is even less. In these cases, one has to weigh the potential advantages of desensitisation and subsequent transplantation with negative crossmatch using the original direct donor.18
Table 2 lists the proposed selection of initial transplantation types in case of donor-recipient incompatibility.
CROSSOVER TRANSPLANT PROGRAMME IN SPAIN
This type of procedure started in Korea and subsequently spread to the U.S. and Holland.16,19,20 The programme was established in Spain in 2009 under the auspices of the ONT, and to date only one procedure between two pairs has been performed.
The regulations that establish crossover procedures were published in 2009. They envisage the creation of a single registry for donor-recipient pairs by the ONT, the appointment of reference centres for performing transplants and the requirements these centres must meet in order to be considered reference centres, procedures for registering pairs in the programme and, lastly, a highly objective scoring system for selecting pair exchanges. The programme also includes the possibility of simultaneous exchange between three or more pairs.
When a centre has a donor-recipient pair who meets the criteria for acceptance into the national crossover transplant programme but the centre has no authorisation for performing the crossover transplant, they can send the pair to the authorised centre they deem most appropriate. They should preferably choose one inside their own autonomous region. Therefore, the inclusion of pairs can be performed anywhere in Spain and is not a restricted treatment.
POSITIVE CROSSMATCH DONATIONS
Early transplant failure due to antibody-mediated acute rejection has been an infrequent phenomenon since the introduction of systematic crossmatches (XM). Nevertheless, patients with negative complement-dependent cytotoxicity (CDC) XM against their (living or cadaveric) donor, occasionally experience hyperacute rejections. Techniques for detecting donor-specific anti-HLA antibodies (DSA) have been refined and made more sophisticated, especially with the introduction of flow cytometry (FC) and solid phase assays. These tests have increased sensitivity and specificity for detecting both class I and II anti-HLA antibodies.21-23 The interpretation of the crossmatch is increasingly complex. The presence of a current positive CDC-XM is considered a contraindication for transplant. The presence of a negative CDC-XM but positive FC-XM complicates decision making, but this is now considered to carry a high risk of early humoral rejection and desensitisation treatment is therefore recommended.24 Studying DSA by solid phase assays helps to better determine whether there are specific antigens against the donor (single antigen).
It is currently possible to perform transplants in patients with DSA and positive crossmatch using desensitisation techniques. There are various desensitisation protocols that usually employ plasmapheresis (PP) or immunoadsorption (IA) combined with intravenous immunoglobulin (IvIg25,26), using different doses and frequencies.17 In recent years, the use of rituximab has been more popular for improving treatment efficacy.27,28 Its dosage and frequency are quite similar to those described above for ABO-incompatible transplantation. As with this type of patient, immunosuppression should be especially potent.29
Table 1. Advantages and disadvantages of crossover and ABO-incompatible transplantation
Table 2. Initial selection in case of incompatible transplant
Figure 1. Grounds for rejecting living-donor transplantation (2000-2008). Fundación Puigvert