La esperanza de vida del niño con enfermedad renal terminal (ERT) depende de un trasplante funcionante (trasplante 63 años frente a diálisis 37 años). El receptor pediátrico es muy adecuado para un injerto de donante vivo, y las contraindicaciones son muy escasas. La posibilidad de evitar la diálisis, elegir el momento del trasplante, proporcionar una buena masa renal, con mínimo tiempo de isquemia fría y mejores identidades en muchos casos hacen del trasplante de donante cadáver una elección idónea. La supervivencia del injerto de donante vivo a largo plazo es significativamente mejor que la de donante cadáver (donante vivo 81,3% frente a donante cadáver 60,8% a 10 años). La vida media calculada de donante vivo en receptores de edades comprendidas entre 2 y 5 años es de 27 años, por lo que es el donante idóneo en menores de 5 años. Los adolescentes (12-17 años) tienen una excelente supervivencia del injerto precoz, pero la peor de todas las edades a largo plazo. Episodios tardíos de rechazo tardío asociados a incumplimiento terapéutico son los factores encontrados en todas las series publicadas. Sin embargo, el trasplante con donante vivo prediálisis tiene una supervivencia del injerto a 10 años del 70%. Los resultados con donante vivo no emparentado en receptores pediátricos son de difícil interpretación.
The most important factor in life expectancy for children on renal replacement therapy (RRT) is to have a functioning graft when they reach adulthood (63 years on transplantation vs 37 years on dialysis). The pediatric recipient is very suitable for a living donor transplantation (LDT), with few contraindications. There are several reasons that make LDT the most recommended RRT in children: pre-emptive transplant avoiding dialysis, good renal mass, minimal cold ischemia time, better HLA-matching and the possibility to program the time of surgery. Long term graft survival in LDT is significantly better than in cadaveric donor transplantation (CDT) (81.3% LDT vs 60.8 % CDT at 10 years follow-up). Calculated half-life graft survival for recipients aged 2-5 years reaches 27.5 years in some series, making LDT the ideal option for these children. Adolescent recipients (12-17 years) have an excellent early graft survival, but the worst long term outcome compared with the rest of pediatric population. However, preemptive LDT has a 70% of graft survival at 10 years. Late rejections episodes associated with non-adherence factors are found in all series. Unrelated LDT in pediatric recipients outcome remain unclear.
EVOLUTION OF CHILD TRANSPLANT RECIPIENTS IN ADULTHOOD
The survival of end-stage kidney disease (ESKD) children on replacement therapy has steadily increased over the last two decades, approaching 80% at 20 years.1
Long-term mortality is mainly caused by infections or cardiovascular complications (40% to 50%), and both hypertension and prolonged periods of dialysis are risk factors.1
A recent study based on data from the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Registry found an increase in the number of young adults who underwent renal replacement therapy as a child. The number went from 71 cases per million inhabitants (PMI) older than 18 years in 1985 to 116 in 2004. Life expectancy for patients who reached adulthood with a functioning kidney transplant was 63 years compared to 38 years for those who remained on dialysis.2
The negative effects of ESKD on these patients also affected their cognitive function and educational development, with cumulative periods of dialysis causing the worst of these effects.3
Studies on intelligence, memory, motor skills and academic performance of chronic renal failure (CRF) children on pre-dialysis show significantly worse results when compared to their siblings.4
Functioning kidney transplantation can reverse these cognitive and intellectual development disorders if performed pre-dialysis or soon after starting dialysis. This would then promote cognitive development, academic life and quality of life for the child with ESKD into adulthood.5-7
The psychological welfare of these young adults also depends on a functioning kidney transplant.8
The benefits of kidney transplantation for children with ESKD are widely documented. Transplantation should be undertaken as early as possible, preferably even pre-dialysis, and with a type of kidney that provides the longest estimated average lifespan.
LIVING DONOR COMPARED WITH CADAVERIC DONOR FOR CHILDREN
Children require an ideal renal mass that ensures good renal function, a condition necessary for prolonged graft survival and longitudinal growth.
Cadaveric kidney donations have changed markedly in the last 10 years, with a shrinking percentage of suitable donors (>6 years and <35 years) and a greater increase in older donors and donor who meet expanded criteria. According to data from the Spanish National Transplant Organisation (ONT by its Spanish initials), in the last year only 2.5% of donors were under 15 years old. Therefore it is increasingly difficult to perform a transplant on a child using a suitable cadaveric donor, especially when transplants from donors who are under 15 years of age are preferred.
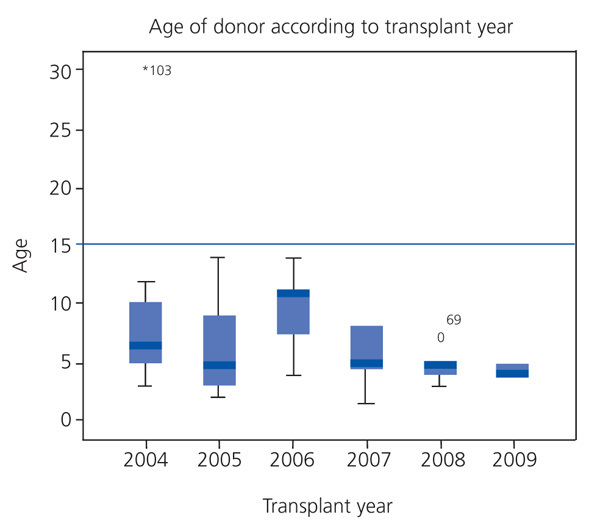
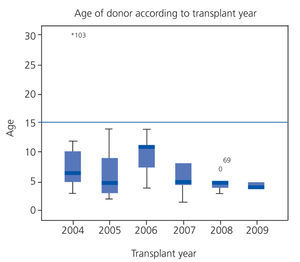
In our hospital, the average age of cadaveric donors has steadily decreased to around four years old in the last two years (Figure 1), making them unsuitable donors in terms of lower renal mass, which can affect long-term survival.
Therefore, paediatric kidney transplantation have to use living-, blood related or not, and ABO incompatible donors in select cases.
The advantages of living-donor kidneys are many:
1. Pre-dialysis transplantation.
2. The ability to choose the right time for transplantation.
3. Good renal mass.
4. Minimum cold ischaemia time.
5. Blood-related donor with three or more donor-recipient HLA matches, in the case of parents.
6. Better long-term graft survival.
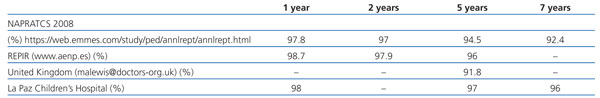
Despite the obvious advantages of living-donor kidney transplantation, data from 2008-2009 show that the use of these donors for paediatric kidney transplantation varies significantly among several countries (Table 1).9-12 Figure 2 shows the number of live kidney transplants performed by paediatric surgeons (in absolute numbers) in Spain between 1991 and 2009.
The biggest drawback has been the potential risks to the donor. However, enough studies now show no appreciable risk of accelerated loss of renal function, greater hypertension or lowered life expectancy.13
Therefore, the first option that should be offered a child with ESKD is replacement therapy.
REQUIREMENTS FOR RECIPIENTS
The requirements for replacement therapy are the same as for cadaveric transplantation, however there are specific issues related to receiving a living-donor kidney.
The recipient and the donor must have a negative crossmatch that ensures the immunological viability of the transplant.
Absolute contraindications are the underlying diseases that recur in a transplanted kidney and cause a high risk of graft loss (oxalosis, atypical haemolytic-uraemic syndrome with risk of recurrence, etc.)
Corticosteroid-resistant nephrotic syndrome (NS) in its genetic form is not a contraindication, given the rarity of recurrence. However, non-genetic corticosteroid-resistant nephrotic syndrome with focal segmental glomerulosclerosis (FSGS) is a relative contraindication due to the high rate of recurrence in the graft, although graft survival may be similar to that in patients with FSGS.14
ABO incompatibility is not currently a contraindication since the transplant can be performed if a specific preparation protocol is undertaken prior to the transplant.15
Failure to comply with treatment during pre-dialysis and dialysis may be a temporary contraindication for living-donor transplantation if the failure is repetitive and serious. The highest risk of graft loss due to non-compliance is in patients older than 12 years (2% between 10-14 years old compared with 0.9% for those under 10 years old; P<.001) according to the United Network for Organ Sharing (UNOS-USA) registry. This negative factor affects graft survival curves, which for adolescents are much lower than for other paediatric ages.16
Therefore, this factor must be taken into account when selecting living donors for this age group at greater risk.
INFORMATION AND DONOR SELECTION
Information process
In the U.S., potential donors for paediatric recipients are, in 80% of cases, the parents.17 Currently, in our department that number is 100%.
This allows information to be absorbed gradually and with enough time so that the process is as complete as possible, both for the donor and the recipient.
The risks of donation in terms of mortality, morbidity, evolution of renal function and hypertension following donation, later pregnancies and psychological aspects must be addressed before starting the examination, based on data from medical literature and experience.17-20
Social and potential job problems for the donor must be taken into account.19 The donor should also be informed of the benefits of their donation,19-21 noting that most studies on the subject found an improvement in their relationship with the recipient.19,20 The non-donating parent showed greater anxiety, stress and psychosomatic and psychiatric symptoms after the transplant than the donor.20
The benefits and risks for the recipient must be explained clearly and simply, emphasising the possibility of pre-dialysis transplantation and the higher long-term graft survival when using a living donor.21,22
The paediatric recipient must be informed from the start. Information must be presented symbolically for the youngest (pictures, stories, simulations, etc.), and cognitively and through dialogue for older children. Coordination between the parents and the physician providing the information is essential for individualising the information for each child.
During the information process, it is important to explore recipient guilt and the fear some children may have about the possible death of the donor parent. All of this requires the support of a professional psychologist in order to carry out an evaluation of both the donor candidate and the recipient.19-21
Starting this information process early is highly recommended and helpful.
Donor Selection
The selection of a donor to start the examination process requires the following:
1. Blood type, ABO and crossmatch.
2. HIV and hepatitis B and C tests.
3. Psychological evaluation of the donor to confirm their motivation, detect possible psychological disorders and determine their capacity to donate.21
4. For recipients with hereditary diseases, potential donors may need previous genetic studies or ultrasounds.
5. Kidney ultrasound showing 2 kidneys.
In this first evaluation for donor selection, one may find that the child and parent are not biologically related. A recent study in the U.S. and Canada found that the prevalence of this situation ranged between 1% and 3%.24 In this study, 23% of professionals were fully in favour of providing this sensitive information to the parents and 24% were completely opposed. This study highlights the importance of studying each case with the maximum confidentiality and respect possible.24
Donor selection is based on:
1. Stated willingness to donate.
2. Better immunological compatibility.
3. Donor age.
4. Psychosocial and occupational impact of the donation on the family.
In order to start the study protocol for child recipients in pre-dialysis and to ensure that the transplant occurs pre-dialysis, donor selection should be started early, when the recipient's glomerular filtration rate is <30ml/min/1.73m2.
RELATED AND UNRELATED DONORS
The trend indicated by the United Network for Organ Sharing (UNOS) is towards increasing the percentage of unrelated living donors, going from 8.2% in 1998 to 23.5% in 2007.25 However, according to data from the North American Paediatric Renal Transplant Cooperative Study (NAPRTCS 2008), only 2.2% out of the 4,410 living donors for paediatric recipients were unrelated.9
Unrelated donors for children recipients are often used in countries like Egypt and Iran, making it difficult to assess the published results.26,27
The NAPRTCS study published in 199828 analysed 38 transplants in children from unrelated living donors (ULD) and compared them to related living donors (RLD) and cadaveric donors (CD). They found a higher rate of acute rejection and lower graft survival at 12 and 24 months in CD than in ULD.28
The recent publication by Dubai29 confirmed that ULD transplants performed on 33 children from different countries (mainly Asian) had worse graft survival at 1 and 10 years than with RLD and CD. Epstein-Barr, cytomegalovirus and varicella zoster virus infections were four times more frequent.29
Although unrelated living donors should be considered for paediatric recipients, acceptance criteria must be well established and regulated.30,31
IMMUNOSUPPRESSION
The initial immunosuppression protocol for transplantation with a living donor does not differ from that of the cadaveric donor, with immunosuppression starting 3-4 days before the transplant.
Baseline immunosuppression may differ from that of the cadaveric donors in the following cases:
1. Identical donor-recipient HLA where immunosuppression can be reduced to monotherapy with the possibility of reaching tolerance and withdrawing monotherapy completely.
2. Tacrolimus monotherapy is possible, preferably with a living donor.32
3. In 90% of living-donor kidney transplants, steroid protocols were only used by the recipient for the first week post-transplant.33,34
Therefore, there is the possibility of reducing long-term immunosuppression when receiving a transplant from a living donor.
SHORT- AND LONG-TERM RESULTS
There are no significant differences in patient survival compared to cadaveric kidney transplantation. In our study, the overall survival rate for 72 child transplants from living donors since 1994 was 98.3%, compared to 96.1% from cadaveric donors. Only one patient died and this was due to leukaemia. The NAPRTCS (2008) data provide similar results: 93.8% at seven years for living-donor recipients and 90.7% for cadaveric-donor recipients.
Paediatric series have shown that donor age influences the long-term outcome of the graft, with younger donors having a worse prognosis. According to the NAPRTCS, survival for cadaveric kidney transplantation at five years is 48% with donors younger than 2 years old, 66% for those between 2 and 17 years old, 71% for 17 to 49 years old and it declines to 57% for those older than 50 years. In our series, we observed that 5-year graft survival was 10% higher in children transplanted using donors older than 10 years of age than in the group using donors under 5 years of age.
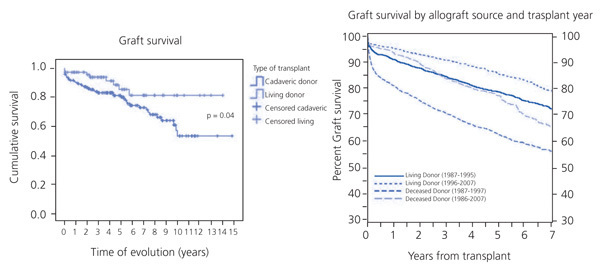
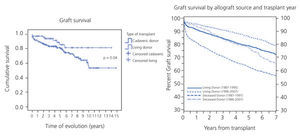
The average age of living donors in our hospital was 39.5±7 years (median: 38.5 years) with a graft survival rate at 1, 5 and 10 years of 97.25%, 85.3% and 81.3%, respectively. There was a significant difference compared with kidney transplantation from cadavers, where the donor age was 9.3±9 years (median: 6 years), with survival rates for the same time periods of 90.4%, 80.1% and 60.8%, respectively. This difference in graft survival became especially noticeable at 4-5 years of transplant evolution, which was similar to data from NAPRTCS (Figure 3). The UNOS series for patients between 2 and 20 years old also noted a 12% improvement in graft survival at five years with living-donor transplantation when compared with cadaveric donors.16 Table 29,16,32,33 shows the results for graft survival from various paediatric series.
When we analysed the loss of grafts in an attempt to assess risk factors, we observed that in our series 11.1% of living-donor grafts were lost compared to 25.9% of cadaveric donors, a statistically significant difference (P=.009). The average age at failure of living-donor transplants was 15.2±5.6 years, as opposed to 14.3±4.5 years for cadaveric donors. We also assessed the average age at transplant, which was 12.4±4.9 years for living-donor recipients and 10.9±4.9 years for cadaveric-donor recipients. If we discard immediate losses, most losses occurred in adolescents and, in 80% of cases, were due to immunological reasons related to poor treatment compliance.
If we study graft survival rates according to recipient age (younger and older than 13 years), survival at one year is similar in both groups. At five years however, the rate is 89.8% in those younger than 13 years and 76.5% for those older than 13 years.
At present, the cumulative incidence of acute rejection has decreased both for living donors and cadaveric donors, reaching similar levels in both groups. However, it is persistently higher than in adult series. In the 2000s, NAPRTCS found an incidence of acute rejection of 26.2% for living-donor transplantation, compared to 23.5% for cadaveric donors. However, our series had a higher incidence of cumulative acute rejection in cadaveric-donor transplantation (29.2%) compared to living-donor transplantation (21.2%), although the differences were not significant.
ADOLESCENT RECIPIENTS OF LIVING-DONOR TRANSPLANTS
Adolescents make up a significant proportion of kidney transplant patients. According to UNOS registry data, recipients between 12 and 17 years old constituted 66% and 57% of the 893 and 796 kidney transplants performed in 2006 and 2007, respectively.25
The needs of these patients are different from those of younger children due to the many changes that occur in this period. Over the past 10 years, publications from the relevant registries in US,34-36 Australia and New Zealand,37 as well as the experience of centres of excellence such as the University of Minnesota (USA),38 have shown similar data on this issue:
1. Adolescents (12-17 years) have excellent survival at one year and the worst survival of all age groups at five years and ten years after transplantation, similar to those older than 65 years of age.34-36
2. The estimated mean graft life for adolescents is 7 years, while for those under two years of age, the mean graft life is 18 years.36
3. The mean graft life for paediatric living-donor recipients at the University of Minnesota was 21.3 years, and 27.5 years for recipients between 2 and 5 years old.38
According to all the publications, poor results in adolescents are associated with a lack of treatment compliance, which triggers late acute rejection episodes with poor response to anti-rejection treatment and the development of chronic injuries.34-36,39
These data led some paediatric transplant centres to place adolescents with poor treatment compliance on waiting lists longer with the hope of improving their attitude towards compliance.
An Australian study confirmed that survival at 5 and 10 years was significantly lower in adolescents (65% and 50%, respectively) compared to recipients aged two to ten years, who had survival rates of 74% and 58%, respectively.37 The most important aspects of this study are that waiting time on dialysis is an independent risk factor for living-donor graft failure (ratio 0.53, P=.03) and that the living-donor graft survival in adolescents transplanted during pre-dialysis is 82% and 70% at 5 and 10 years, respectively. This study supports pre-dialysis living-donor transplants in adolescents.37
No solution has been found to all these problems. Given the complexity of adolescence, the solutions will require further research.
The excellent review by Smith et al36 on adolescents as recipients of solid organ transplants serves as a guide for improving understanding of the problem and articulating some practical measures for reducing non-compliance in these patients.
Treatment non-compliance
Treatment non-compliance of up to 64% has been reported in kidney transplantation in adolescents, which causes graft loss in 12% to 34% of cases.40 Multiple efforts have been directed towards finding out which risk factors lead adolescents to non-compliance. Mental disorders (depression and anxiety), lack of family support, low self-esteem prior to adolescence, side effects of immunosuppression, particularly cosmetic effects, and the complexity of the treatment regimen, are found repeatedly in studies on risk factors for non-compliance.36,40
It is essential to understand the particular conditions of this age group and assess and incorporate them into the treatment process26:
1. The peculiarities of the physical, emotional and psychological development of the adolescent. It is important to be aware that the brain’s executive function (organisation, planning, self-regulation, selective attention and inhibition) is the last acquisition in the adolescent brain development.36
2. The needs of puberty and the emotional development (autonomy and identity) should be attended to and the adolescent should be supported in order to encourage their growth.
3. Identify the risks inherent to their sexuality and use of contraceptives and their effects on immunosuppression, hypertension, etc.
4. Periodically measure compliance with treatment (especially levels of immunosuppression, which fluctuate over time) and talk openly with the adolescent about compliance and its consequences, seeking their commitment and participation in the process.
5. Provide psychotherapy for depression and anxiety disorders, from which 5% to 14% of adolescents with solid organ transplants suffer,36 and for eating disorders and drug abuse.
Currently, no method has been shown to be effective in improving treatment compliance.
However, various interventions have been suggested that are worth trying:
1. Reduce as much as possible the number and frequency of medications.
2. Identify recipients at risk and provide psychotherapy and educational intervention about medication, visits, risks they are exposed to, etc.
3. Increase visits with higher-risk patients or at specific times when fluctuations in immunosuppression levels are confirmed.
4. Avoid cosmetic side effects (no steroids or early withdrawal, and when possible, avoid cyclosporine, minoxidil, etc.)
5. Talk openly with the adolescent about non-compliance, provide them with information and explain the measures being taken and their reasons.
6. Listen to their requests and attend to their need for autonomy, mental health and vocational aspirations.
Changing the poor long-term outcome of kidney transplantation in adolescents requires strategies that are individualised and multidisciplinary. It also requires group research that has the means and sufficient patients to make the investment in human resources and materials necessary to carry them out practical and cost-effective.
LIVING-DONOR PAEDIATRIC RECIPIENTS AND VENA CAVA THROMBOSIS
Vena cava thrombosis in children with ESKD is a rare but very complex situation with high risk for the transplanted graft. In this situation, a living-donor may be a good choice.
For this situation, the following is required:
1. Detailed studies of abdominal vasculature.
2. The surgical and nephrology teams must carefully select a donor: paediatric or adult kidney, living or cadaveric.
3. The surgical team must carefully select the location of anastomosis in the donor and possible complications.
The literature has reported 16 cases41 of which six were performed with living donors and ten with cadaveric donors, the majority with adult donors. The surgical results were excellent.
Of the 16 cases published, four were from our hospital, with two of these performed with living donors and the other two with cadaveric donors. Orthotopic implantation was necessary in 3 cases.42
Living donors are an option for children with inferior vena cava thrombosis, although parents and recipients (older than 16 years) must accept, along with the team of physicians involved, the greater risk that this type of transplant involves.
Table 1. Use of living donors for paediatric transplantation
Table 2. Survival of kidney transplant patients. NAPRATCS 2008
Table 3. Graft survival in various paediatric series
Figure 1. Evolution of donor age in cadaveric kidney transplantation in La Paz Children's Hospital (2004-2009)
Figure 2. Live kidney transplants performed by paediatric surgeons (absolute numbers) in Spain. 1991-2009
Figure 3. Actuarial graft survival curve in La Paz Hospital and the NAPRTCS (North American Pediatric Renal Transplant Cooperative Study)