El objetivo del estudio consiste en evaluar el riesgo de pérdida del injerto. Deben identificarse en el receptor los aloanticuerpos donante-específicos y determinarse las incompatibilidades HLA entre receptor y donante. Para determinar los aloanticuerpos existen diferentes métodos que tienen diferente sensibilidad y diferente valor pronóstico, unos determinan un alto riesgo de rechazo hiperagudo, otros un aumento en el riesgo de pérdida de injerto en retrasplantes. Determinaciones en la primera fase del estudio pretrasplante: a) tipificación HLA del receptor y de los posibles donantes; b) aloanticuerpos por citotoxicidad dependiente de complemento frente a panel (PRA-CDC) y cribado de aloanticuerpos anti-HLA por fase sólida; c) en enfermos sensibilizados, puede ser útil identificar las incompatibilidades aceptables mediante una determinación de antígeno aislado en fase sólida y la evaluación del «crossmatch virtual» (VCM). Estudio pretrasplante inmediato (10 días): a) prueba cruzada linfocitaria por citotoxicidad (CM-CDC) entre receptor y donante; b) prueba cruzada linfocitaria por citometría de flujo entre receptor y donante (FCCM) especialmente indicada en el retrasplante. Permite también descartar autoanticuerpos IgM. Desensibilización de los receptores: evaluar la necesidad real y las posibilidades de éxito antes de iniciar un tratamiento. Monitorización inmunológica postrasplante: determinación de aloanticuerpos si es necesario para: a) diagnóstico diferencial de episodio de rechazo corticorresistente con componente humoral, y b) como marcador de probabilidad de la reducción de supervivencia a largo plazo. Epílogo: Debe valorarse la historia de alosensibilización del receptor. El crossmatch por citotoxicidad pronostica un alto riesgo de rechazo hiperagudo y se considera una contraindicación. El crossmatch por citometría indica un aumento de riesgo de pérdida al año del injerto, bajo en el primer trasplante (>10%), pero mayor en el retrasplante (>30%). El VCM por fase sólida positivo indica un incremento del riesgo de un episodio de rechazo mediado por anticuerpos (del 5 al 55%), pero no contraindica necesariamente el trasplante.
The objective of the study is to evaluate the risk of graft failure. The presence of donor specific alloantibodies and the HLA incompatibilities between donor and receptor must be identified. There are several methods to identify alloandibodies that has different sensitivity and different Prognostic Value. Some define a high risk of hyperacute rejection, others an increase in the risk to loss the graft in defined subgroups. First steps of the pretransplant study identify: a) HLA typing of the receptor and available donors; b) alloantibodies by Complement Dependent Cytotoxicity against Panel (PRA-CDC) and screening of alloantibodies against HLA by Solid Phase; c) in sensitized receptors it can be useful to identify acceptable incompatibilities using Single Antigen Solid Phase technique and to evaluate the «Virtual Crossmatch». Pretransplant study (10 days): a) crossmatch by Citotoxicity (CM-CDC) between receptor and donor; b) crossmatch by Flow-Cytometry (FCCM) between receptor and donor specially indicated in the retransplant. Useful also to discard IgM auto-antibodies. Receptors desensitization: the necessity and success probability of desensitization should be evaluated before treatment. Post-Transplant Monitoring: identify alloantibodies for: a) the differential diagnostic of corticorresistant rejection episodes with humoral component, and b) as a marker of long term reduced graft survival probability in the long term. Final remarks: Evaluation should consider the allosensibilization history of the receptor. The cytotoxicity crossmatch indicates a high risk of hyperacute rejection and is considered a contraindication. The Flow Cytometry crossmatch indicates an increase in the probability to loss the graft in the first year that is low for first transplants (>10%) but higher for retransplantation (>30%). The virtual crossmatch by solid phase indicates an increase in the probability to have an antibody mediated rejection (from 5% to 55%) but did not contraindicate always the transplant.
INTRODUCTION
Immunological studies of donor-recipient pairs are aimed to quantify and minimise the likelihood of graft loss in the short and long term. The risk of graft loss can be assessed pre-transplant and post-transplant by using various immunological tests. The results of these tests can help avoid very high-risk transplants and also provide the appropriate type of immunosuppression treatment for each level of risk.
The classic lymphocyte complement dependent cytotoxicity crossmatch or CDC crossmatch has a high positive predictive value (PPV) for graft loss (80%) and a positive result for this test has traditionally been a contraindication for kidney transplantation. Other tests have less resounding prognostic value and positive results indicate increases in graft loss risk that vary between 10% and 30% at one year.
The decision to perform a particular transplant depends on: 1) the level of acceptable risk, 2) the alternatives for a specific patient and 3) the diagnostic and treatment tools available.
The most appropriate type and intensity of immunosuppression must be selected through an assessment of current data and the patient's alloresponse history.
Transplant success depends on preventing recipient alloresponse due to differences in major histocompatibility antigens of the transplanted organ, mainly the HLA system. The alloresponse will be more intense the greater the difference between donors and recipients.
Most available immunosuppressants can induce graft acceptance, but are generally ineffective on alloantibody-producing plasma cells.
TECHNIQUE SELECTION
The objectives of most techniques used are:
1. Evaluate the incompatibilities the recipient will detect in the donor cells.
2. Identify the presence in the recipient of alloantibodies against HLA polymorphisms of potential donors.
3. Identify whether recipient alloantibodies react against polymorphisms expressed in the proposed donor.
4. Identify the type of immunoglobulin (IgM or IgG) and target (HLA-I, HLA-II, or no HLA).
The different techniques for identifying alloantibodies are mostly similar, however the targets for each technique are different (viable cells or purified HLA antigens). The techniques use different methods (cytotoxic capacity or IgG markers), have different levels of sensitivity and are interfered with by different elements (see Appendix 1).
In practice, each method serves to predict different events and/or has different prognostic values. It is the joint evaluation of different tests that enables actual risk assessment.
In each technique, one should consider the type of event to be predicted and the positive predictive value (PPV) or negative predictive value (NPV) of its result.
There are few prospective studies that assess the prognostic values of the most recently introduced tests regarding well-defined clinical events. Their assessment with respect to reference techniques may lead to some confusion because the new techniques may offer greater sensitivity. Everything indicates that this increase in sensitivity does not have the same prognostic value as the reference technique (for example, cytotoxicity) and may also predict different events than those of this reference technique.
The choice of techniques should be based on the clinical usefulness of the information provided. However, the economic cost and logistical and staffing difficulties should also be considered, avoiding unnecessary use of resources whenever possible.
To ensure efficiency and reliability of the tests, the laboratory must continuously monitor its processes and results. Monitoring is guaranteed by the accreditation of processes and quality control of results, evaluated by external organisations of experts on histocompatibility (for example, the accreditation programme of the European Federation for Immunogenetics [EFI] and the American Society for Histocompatibility and Immunogenetics [ASHI]).
INDICATION AND INTERPRETATION OF THE VARIOUS TESTS IN LIVING-DONOR TRANSPLANTATION
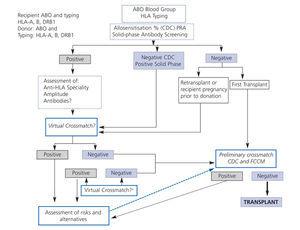
The selection process for a donor who is immunologically compatible with a kidney recipient is gradual. The procedure to be followed will be based on the test results (Figure 1).
First phase of the pre-transplant study
HLA typing of recipients and potential donors
Recipients and donors should be typed at least for HLA-A, HLA-B and HLA-DRB1 at the antigen level (first two or three digits). DNA-based HLA-typing techniques for DNA are highly recommended for their reliability and reproducibility. For HLA-A and HLA-B typing, serology may be acceptable.
If anti-HLA-C, HLA-DQ or HLA-DP antibodies have been identified in the recipient, then it may be useful to expand donor typing to these loci (see virtual crossmatch)
Allelic typing of the donor (four or more digits) may be indicated in special cases where there are antibodies against only some alleles (4 digits) of the same antigen group (2 digits) (for example, A2402 positive, A2403 negative).
Usefulness of HLA typing
1. Helps determine the probability of long-term graft survival. Survival at 10 years for HLA-identical grafts: 73%; survival of non-identical grafts from 64% (one incompatibility) to 53% (six incompatibilities).1
2. Helps evaluate the likelihood of a negative crossmatch, knowing the percentage of CDC-PRA and the specificity of the recipient's antibodies. For highly sensitised patients, having an HLA-identical sibling is sometimes one of the few opportunities for transplantation.
3. Makes it possible to evaluate the so-called virtual crossmatch (VCM).
4. Helps identify donor-specific alloantibodies after transplantation using solid-phase techniques.
5. Makes it possible to avoid repeating the same incompatibilities in future transplants.
Alloantibodies by complement dependent cytotoxic panel reactive antibodies (CDC-PRA) testing
Alloantibody testing should be performed every three months in all patients able to be transplanted2 and 15 days after each sensitising event (transfusion, graft loss and after pregnancy).
This sequential study helps reveal antibodies that may have been identified in the past but that may not have been detected at the time of transplantation. These antibodies may have generated memory lymphocytes that are easily reactivated, so adapted immunosuppression will be needed.
Three types of information are obtained from the CDC-PRA test:
1. Whether the recipient has alloantibodies or not.
2. The reactivity percentage, which predicts the likelihood of a positive lymphocyte crossmatch.
3. Identify, in some cases, which antigens these alloantibodies react against. For donors who have these antigens, this information helps predict a very high probability of a positive lymphocyte cytotoxicity crossmatch.
Solid-phase anti-HLA alloantibody screening
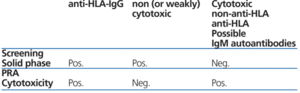
Should be conducted: 1) when previous uncontrolled sensitising events have arisen or cannot be ruled out, 2) when autoantibodies are suspected and there is a need to rule them out in a patient with positive CDC-PRA (Figure 1). Indicates presence or absence (positive/negative) of type IgG anti-HLA antibodies against anti-HLA-I (HLA-A, B, C) alleles and/or anti-HLA-II (DR, DQ, DP) and, in some kits, anti-MICA.
Has greater sensitivity than panel-reactive antibody detection (PRA) in complement dependent cytotoxicity (CDC) test.3,4
If an anti-IgG is used as a secondary antibody, type IgM antibodies are not detected.
By using purified HLA antigens, non-anti-HLA antibodies are not identified.
If anti-HLA antibodies are not revealed by cytotoxicity but are detected by solid phase, it is highly recommended that more sensitive crossmatch techniques be used, such as flow cytometry or virtual crossmatch (VCM) to better define the risk for these patients.
IgM autoantibodies do not contraindicate a transplant, as long as it is possible to rule out the presence of type IgM anti-HLA antibodies induced by a recent transfusion, which can affect graft survival.
Virtual lymphocyte crossmatch (VCM)
This test is indicated in patients who are candidates for retransplantation, women who have previously been pregnant and those with positive results in the solid-phase screening but negative for CDC.
This technique lets the clinician know precisely and with high sensitivity whether a recipient has IgG antibodies against each of the HLA system antigens.
Antibodies against HLA class I (A, B, C) molecules and HLA class II (DR, DP, DQ) molecules can be identified. The reagents are expensive.
Knowing the donor type will help make a very rough estimate of donors that will not react with the recipient's antibodies. This approximation is known as VCM.
VCM allows one to define, even in patients with antibodies against many alleles, the so-called "acceptable incompatibilities", and is a valuable tool for identifying potential donors for highly sensitised recipients.
As the result of this test is a numerical value (semiquantitative), it is important to distinguish the lower threshold of sensitivity of the technique from the threshold that predicts a particular clinical event. The setting of these thresholds is under evaluation; therefore if antibodies are also detectable by other better evaluated techniques, such as cytotoxicity crossmatch or cytometry, the prognostic value of these prevails.
In the event that the only positive result is that of the positive VCM, with other techniques being negative, this will indicate a 55% probability of an antibody-mediated rejection episode in the first year versus a 5% probability in the case of a negative VCM. Forty-five percent of recipients who do not suffer a rejection episode will have a survival similar to those with negative VCM; only those that suffer from a rejection episode will have survival reduced by 20% at 5 years.5
Anti-HLA-C,6 anti-HLA-DQ7 and anti-HLA-DP8 antibodies have been associated with rejection. If the recipient has antibodies against these antigens then it may be appropriate to know the donor's alleles at these loci, although the pretransplant prognostic value of these antibodies is not well defined.
VCM has a high negative predictive value for cytotoxicity crossmatch. Its PPV is lower, i.e., it detects antibodies that are not revealed by cytotoxicity.9
It follows that a positive VCM, on its own, does not imply that a transplant is necessarily contraindicated. It is, however, a red flag indicating the need for a thorough evaluation, careful monitoring and immunosuppression aimed at controlling alloantibody production.
Usefulness and interpretation of the preliminary crossmatch
The preliminary crossmatch is indicated in recipients at risk for positive crossmatch. The purpose of this preliminary study is to avoid expensive studies on donors at high risk of being rejected for immunological reasons in the immediate pretransplant tests (10 days).
The crossmatches to be used for the immediate pretransplant study must be included.
Recipients at risk for positive lymphocyte crossmatch are those who: 1) have a positive CDC-PRA or solid-phase PRA, 2) are recipients of retransplants including those without alloantibodies detected by CDC-PRA, and 3) women who have been pregnant prior to their candidacy as donor (Figure 1).
Immediate pre-transplant study (10 days)
Lymphocyte CDC crossmatch between donor and recipient
This test must be performed before any kidney transplantation.
A positive complement dependent cytotoxicity (CDC) crossmatch using T-lymphocytes or total lymphocytes at 22ºC±5ºC have a PPV for graft loss in the first 48 hours of 80%, which therefore contraindicates transplantation.
Transplantation may not be contraindicated if there is evidence that the positive result was due to anti-IgM autoantibodies. This requires that: 1) the positive becomes negative after serum treatment with DTT, 2) there is no evidence of a sensitising event in the last 15 days and 3) the determination of solid-phase anti-HLA alloantibody screening (Luminex) is negative in serum that was CDC-PRA positive. Autoreactivity can be confirmed using evidence of autoimmune diseases (SLE, RA, PBC, etc.) or previous or simultaneous determination of a positive autologous complement dependent cytotoxicity (CDC) crossmatch, which becomes negative after serum treatment with DTT.
At the very least, it must include sera with the highest CDC-PRA percentages over the last two years and those subsequent to sensitising elements. The current serum should be studied in duplicate, undiluted and untreated, and at least at one dilution (EFI standards).
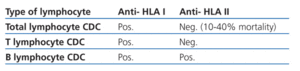
A positive B-cell negative T-cell crossmatch may be caused by three conditions: a) presence of anti-HLA-II antibodies b) presence of low-titre anti-HLA-I antibodies detectable only in B lymphocytes since these express greater amounts of HLA-I than T lymphocytes and c) presence of specific B lymphocytes autoantibodies.
Temporal criterion: crossmatch that was previously positive and is now negative. The contraindication criteria apply generally to serum on the day of the transplantation and those of the last two years. For sera taken more than 2 years pre-transplant, there is evidence that patients with PRA>80% undergo the risk of additional loss of 20% over patients with negative historical sera.10
Lymphocyte flow cytometry crossmatch between donor and recipient (FCCM)
This test should be performed on patients who are candidates for retransplantation, on women with previous pregnancies, those with positive results in the solid-phase screening but negative for CDC and is recommended for all living-donor transplants.
This test makes it possible to determine the graft loss risk at one year, especially in retransplants.
It rules out positives in the CDC crossmatch that do not contraindicate transplantation (for example, IgM autoantibodies).
It provides semiquantitative values for the amount of existing antibodies, evaluating the change in mean fluorescence channel, and is an easy, complete and accurate method for identifying alloantibodies against all donor HLA-II antigens.
It is especially indicated if alloantibodies are detected by solid phase but are not evidenced by cytotoxicity.
If the simultaneous complement dependent cytotoxicity (CDC) crossmatch is positive then the corresponding criterion will be applied to the cytotoxicity test.
If the complement dependent cytotoxicity (CDC) crossmatch is negative and the cytometry crossmatch is positive, the probability of graft survival at one year is slightly lower than those of grafts with negative cytometry crossmatch (they may be equal in some centres):11 a) first transplant: 10% lower and b) retransplants: 30% lower.
Thus, detection of antibodies undetectable by complement fixation does not predict a hyperacute rejection, but does predict lower graft survival, especially in retransplants.
The assessment of a positive B-cell negative T-cell crossmatch remains controversial. Positive B-cell cytotoxicity has not historically been considered a contraindication for first transplants in many centres. One should not forget that some anti-B cytotoxicity antibodies are actually IgM-type autoantibodies that do not produce positive reactions in cytometry. However, anti-HLA-II IgG antibodies due to a previous graft have been associated with rejection episodes and have been considered a relative indicator of poor prognosis in retransplantation.12 Anti-HLA-DR, anti-HLA-DQ and anti-HLA-DP IgG antibodies have been associated with rejection. Therefore, when faced with a positive B lymphocyte cytometry crossmatch, the decision for transplantation must be individualised, assessing all sources of information as a whole.
Recipient desensitisation
There is evidence that it is possible to reduce pre-existing circulating alloantibodies in some patients to levels where the antibodies are unable to trigger hyperacute rejections. This does not imply that there are no B lymphocytes with the capacity to restart alloantibody production, but the short-term survival of grafts transplanted in some centres under these conditions is acceptable.
We will not discuss the indications or different desensitisation techniques here, which in any case should be done by an experienced team. However, it is important to know and assess the immunological markers that would justify desensitisation or indicate its chances of success that have been recently reviewed.13 One should remember that not all sensitised patients are candidates for desensitisation. We must consider two issues:
1. Who are potential candidates for desensitisation?
2. In which patients would desensitisation likely be successful?
Who are potential candidates for desensitisation?
Recipients with positive cytotoxicity crossmatch (once the autoantibodies have been excluded) are potential candidates for pre-transplant desensitisation.
Retransplantation recipients with positive cytometry crossmatch and negative cytotoxicity crossmatch are potential candidates.
For first transplant recipients with positive cytometry crossmatch but with negative cytotoxicity crossmatch, desensitisation may not be necessary.
For patients who are only positive for VCM, with negative cytotoxicity and cytometry crossmatches, there are currently insufficient data that support the appropriateness of desensitisation.
In any case, the available information should be evaluated as a whole by an experienced team: 1) history of sensitisation levels and sensitising elements, 2) whether the positive results affect T and B lymphocytes or only B, 3) the value of the change in mean fluorescence channel of the cytometry crossmatch and 4) the number of positives and the value of the MFI in the VCM.
In which patients would desensitisation likely be successful?
First, a successful desensitisation needs to be defined: Are we attempting to make the cytotoxicity and cytometry crossmatches negative or only the cytotoxicity crossmatch? It is essential to consider the previous experience of the laboratory and the sensitivity levels for each technique in the external quality controls (for example, Taller Ibérico de Histocompatibilidad [Spanish Histocompatibility Workshop]).
In general, two parameters are used to predict the probabilities of successful desensitisation: a) the last serum dilution that comes out positive in the cytotoxicity crossmatch and b) the change in the mean fluorescence channel of the cytometry crossmatch.
With regard to cytotoxicity crossmatch, many laboratories consider that titres equal to or greater than 1/128 have little or no possibility for desensitisation. Moreover, titres less than or equal to 1/32 are open to desensitisation, with titres of 1/64 being the subject of controversy.
With regard to cytometry crossmatch, the literature is sparse, but one could say that changes in mean channel fluorescence lower than 70 are likely to have a successful desensitisation, while above that level the likelihood is very low. In both cases, the cutoff depends on the sensitivity of the technique used in each laboratory.
Post-transplant immunological monitoring
The deterioration of renal function is frequently the first sign of rejection. Discovering this deterioration using immunological tests is difficult for various reasons: 1) a high frequency of determinations is needed to properly distinguish markers of renal deterioration and 2) if the tests are not antigen-specific, they are frequently modified by infectious episodes. However, alloantibody determinations are useful in two circumstances:
Differential diagnosis of steroid-resistant rejection with humoral component
Faced with an acute rejection episode, it is important to determine if the episode has a humoral component or not. Rejections with a cellular component are mostly sensitive to corticosteroids. Resistance to treatment with corticosteroids is the de facto first sign of an antibody-mediated component. Rejections with a humoral component require specific treatments, such as plasma exchange and interventions on immunoglobulin-producing lymphocytes.
The diagnosis of a humoral rejection is made not only by the type and distribution of infiltrating cells but also by the determination of circulating donor-specific antibodies (DSA). The presence of C4d deposits in peritubular capillaries has, apparently, a positive predictive value lower than that of antibodies.
Clearly identifying the reactivity of antibodies with the donor is logistically difficult because it requires donor cells. These cells may be stored frozen in liquid nitrogen, or the living donor will have to be present for each determination.
The most conclusive DSA test is the flow cytometry crossmatch. Cytotoxicity techniques, even though they may be used, are less sensitive than cytometry. Permanence in situ of the graft helps sequester some of the alloantibodies reducing the circulating antibodies. Thus, the use of the most sensitive techniques available, cytometry and/or solid phase, is recommended.
The sequence of tests must balance the necessary information for making decisions and the available resources. Faced with suspicion of steroid-resistant rejection in:
1. Patients without prior alloantibodies: a) perform a solid-phase HLA-I and HLA-II alloantibody screening, b) if negative then there are no detectable anti-HLA antibodies and in principle a specific donor crossmatch is not undertaken, and c) if they are positive then one must assess whether the antibodies have been induced by the donor, or otherwise it is strictly necessary to confirm that the antibodies are donor-specific by cytometry crossmatch (for example, if there is suspicion of alloantibodies having been induced by other sensitisation sources).
2. Patient with previous alloantibodies by solid-phase assay: a) if their disappearance is to be monitored then a solid-phase HLA-I and HLA-II alloantibody screening may be useful, b) if donor cells are available then a donor-specific cytometry crossmatch may be indicated.
In both situations, and when the crossmatch cannot be performed due to lack of donor cells, a determination by solid phase with single antigen may be considered.
It is important to note that it is only possible to identify alloantibodies against alleles that have been identified in the donor. In other words, if the donor has been typed for HLA-C, HLA-DQ or HLA-DP and the recipient has antibodies against these alleles, the VCM results will only be conclusive if they are positive, but inconclusive if negative because some of the targets present in the graft are unknown.
The single antigen reagents are expensive and indiscriminate requests for them may significantly affect the budget for transplantation programmes.
The participation of alloantibodies in rejection makes its advisable to perform a treatment targeted at reducing them (plasma exchange, administration of intravenous immunoglobulins, anti-CD20, etc.)
Monitoring of treatment can be performed by measuring the decline in alloantibodies: 1) by changes in the mean fluorescence channel of the cytometry crossmatch, 2) by decreasing titres of complement dependent crossmatches if they are positive (for example, from 1/128 to 1/32) and 3) measuring the change in MFI of donor alleles in solid-phase assays that use single antigens.
Alloantibodies as markers of poor prognosis
There is growing evidence that not only acute humoral rejection but also interstitial fibrosis and glomerular atrophy in chronic rejection are more frequent in recipients who have developed ex novo alloantibodies post-transplant. Therefore, before significantly reducing or changing immunosuppression, an assessment of the probability of graft loss may be indicated. When calculating this probability, the determination of alloantibodies is one of the most accepted markers.14 It should not be forgotten, however, that this implies an increase in graft loss risk, but it is possible to find many patients with donor-specific alloantibodies with no clinical symptoms and who will probably not lose their graft in the medium term.15
The presence of class I-anti-HLA antibodies post-transplant precedes, even by years, the development of glomerulopathy.16 The presence of anti-class II antibodies is strongly associated with chronic rejection in living-donor kidney recipients, but it appears that the worst prognosis is associated with the simultaneous detection of anti-HLA-I and anti-HLA-II antibodies.17,18 For this screening, apparently, determination by solid phase is the indicated technique. Later identification of reactivity with the donor may or may not be necessary depending on the circumstances of the recipient (transfusion history, previous alloantibodies, etc.)
EPILOGUE
Allosensitisation data must be evaluated as a whole, also taking into account the recipients' allosensitisation history. Cytotoxicity crossmatch between donor and recipient, at the time of transplantation, predicts a high risk of hyperacute rejection and is considered a contraindication. Cytometry crossmatch indicates a low increase in graft loss risk at one year in the first transplant (>10%), but greater in retransplants (>30%). Solid-phase VCM indicates increased risk of antibody-mediated rejection episodes (from 5% to 55%) but does not necessarily contraindicate transplantation. These risks must be assessed in the context of the patient's general condition and the available diagnostic and treatment resources.
Figure 1. Immunologic evaluation algorithm
Table 1. Identification of anti-HLA-I and HLA-II antibodies. Identification of anti-HLA-I and HLA-II antibodies
Table 2. Identification of immunoglobulin type in CDC
Table 3. Anti-HLA class and type of immunoglobulin by cytometry (with anti-IgG)
Table 4. Interpretation of differences between cytotoxicity and solid phase