Donor protection should always be taken account during the selection and assessment of a living donor. On these terms, the evaluation of a potential donor must include these issues: 1) The donor act is altruistic, consciousness and out of coercion; 2) Life expectancy and quality of life of the recipient will improve after the living donor kidney transplantation; 3) The donor has normal renal function and the potential risk of developing nephropathy in the long term follow up is scarce (familiar nephropathies and other processes that may increase the potential risk for renal disease in the future, like severe hypertension, diabetes, etc must be ruled out). The glomerular filtrate should meet criteria for the normal function corresponding to age furthermore the absence of proteinuria and urine smear is normal; 4) The screening in the donor should contemplate those clinical situations or diseases non related to the kidney function but might elevate the surgical and/or anesthesia risk besides disease transmission to the recipient (as neoplasia or infections); 5) The surgical act is possible without technical difficulties and always performed after a negative result of the crossmatch between donor and recipient. The living donor evaluation process will follow a different schedule based on each particular case and the center facilities. Any case, the mentioned process is divided in two parts: The first one contains an initial screening (using non invasive and low cost tests) that allows discarding contraindications for donation (in both donor and recipient). In a second phase the assessment of the donor varies with donor characteristics. However, a test for renal function is mandatory besides imaging techniques (like angioTC), screening for transmissible diseases and a detailed evaluation for psychosocial aspects preferably made by professional. Moreover Spanish policy on living donation requires a report with information about the consent for donation developed by an independent board (ethics committee) besides the consent for donation given at the civil registry.
En la selección y el estudio del donante de riñón, el principio predominante para el médico debe ser la protección del donante. El estudio de una donación de riñón de vivo debe demostrar diversos aspectos: 1) la donación es libre, consciente y desinteresada; 2) el receptor no presenta contraindicaciones y su pronóstico vital y de rehabilitación mejorará de forma relevante con el trasplante renal de donante vivo; 3) el donante tiene riñones normales y el riesgo de desarrollar nefropatía a largo plazo es reducido. El filtrado glomerular debe estar por encima de un nivel mínimo en función de la edad y no deben existir proteinuria o alteraciones del sedimento. Deben descartarse nefropatías heredofamiliares y procesos o alteraciones que incrementen el riesgo de nefropatía a largo plazo (enfermedades sistémicas, hipertensión arterial severa, diabetes, etc.); 4) el donante no debe presentar otras enfermedades o alteraciones que puedan incrementar el riesgo quirúrgico o anestésico o transmitirse al receptor (cáncer, infecciones); 5) el trasplante es posible técnicamente con un riesgo aceptable: anatomía apropiada en donante y receptor, compatibilidad ABO y prueba cruzada negativa (excepto si se van a aplicar técnicas preparatorias especiales). El estudio del donante se organizará en función del caso particular y de las facilidades disponibles en el centro. En cualquier caso debe comenzar por una fase de cribado con estudios poco invasivos y costosos, que descarte contraindicaciones elementales por parte de donante y de receptor. En una segunda fase se amplían las exploraciones en función de las características del donante, si bien en todo caso deben incluir la comprobación de la función renal, estudio de imagen mediante angiotomografía axial computarizada, cribado de infecciones trasmisibles y de cáncer y un examen más detallado de los aspectos psicosociales, a ser posible por parte de personal especializado. La normativa española exige la emisión de un informe por parte del Comité Hospitalario de Ética y la declaración de voluntad del donante ante el juez del Registro Civil.
INTRODUCTION
Increasing attention has been focussed on the assessment and selection process of the living kidney donor as living-donor kidney transplantation (LDKT) has become an essential option in chronic kidney disease (CKD) replacement therapy. Various scientific associations and highly-experienced health-care providers,1-7 including some Spanish groups,8-10 have provided Recommendation guidelines. It is important to mention the international consensus of experts reached in Amsterdam in 2004,11 as it represents an approach that can be applied to different economic, social and health care contexts. These Guidelines are based on these Recommendations and more recent guidelines.5 However, the great variability in the medical practice between countries and between hospitals in the same country12-15 has been demonstrated repeatedly. This may be due, in part, to the low level of evidence of the recommendations.16 All of the studies on the long-term impact of donation are retrospective and they are usually based on single-centre analysis.17 Even though there are now some large-scale studies (with limited data)18 and meta-analysis on specific aspects,19-21 the level of evidence reached is always below 3a – CEBM. The conclusions are usually limited due to the use of small samples, the low follow-up level, unclear methods for measuring the objectives (blood pressure or glomerular filtration rate, for example), and the large variability between the studies. With the exception of a small number of studies that have used family members or potential donors as control subjects (with small samples), the majority of studies have used the general population as the control group. It is debatable if donors and the general population can be compared given the meticulous selection process the former has to undergo.16 In any case, these medical studies provide little guidance on donor assessment methods and make the recommendations difficult to follow. All of the recommendations are based on evidence level III-IV.5
STUDY OBJECTIVES
The general objective of the study on the donor-recipient pair with a view to a LDKT is to guarantee, within reason, that the starting conditions are in place in order to achieve the expected results: to significantly improve the recipient’s survival and recovery prognosis, while causing little or no harm to the donor. The main aim of the doctor must be to protect the donors, not to cause them any harm. LDKT will very rarely be the only viable option available to the recipient to survive in the short/medium-term. Therefore, it is not usually justified to subject the donor to any significant risks. However, difficult situations are quite often considered when it is not certain whether the expected results in either or both of the objectives of the transplantation will be achieved: debatable improvement for the recipient, or small or unknown increased risk for the donor. In such cases, the donor-recipient pair must take part in the decision and then, it must be later approved by the external opinion of the hospital’s Ethics Committee.
The aim of a comprehensive study of a living kidney donation is to check for the following requirements:
1. The donation is made freely, consciously and willingly. This implies, among other things, that donors: a) do not have any cognitive or emotional disorders; b) have sufficient intellect and level of understanding to be able to comprehend the information on the risk and benefits; c) are not being forced into the decision, and d) are not looking for any material reward for the act.
Other articles in this issue have examined in detail the ethical and psychosocial aspects of LDKT.
2. Donors have two normal kidneys and are at a low risk of developing a kidney disease in the future: a) they have normal renal function and no significant abnormalities are found during the functional and structural tests on the kidneys; b) they have no family history of kidney disease that may develop later in life, and c) there are no disorders or abnormalities that increase the risk of kidney disease, for example, systemic diseases, severe high blood pressure (HBP) or diabetes.
3. Donors do not have any other diseases or disorders that may: a) increase the surgical or anaesthetic risk; b) be affected by a lower kidney reserve, or c) be transmitted to recipients, such as cancer or infections.
4. Recipients are accepted for transplantation: they have no contraindications, and their survival and recovery prognosis is reasonably good and they will improve significantly with LDKT.
5. The transplant is technically possible with an acceptable risk: a) suitable vessels and urinary tracts (donor and recipient), and b) they are compatible: ABO compatibility and negative crossmatch (unless special preparatory techniques are going to be used).
GENERAL ORGANISATION OF THE STUDY
Very different approaches can be used depending on the characteristics of the donors, how fast the study needs to be carried out, facilities and local waiting times for the tests. Therefore, the studies can be planned all at the same time from the beginning in very clear cases, especially if a quick decision is expected. The order in which the tests are performed must also change depending on each case, for example, if donors have specific diseases that need approval by other specialists, further tests must not be performed until this approval is granted.
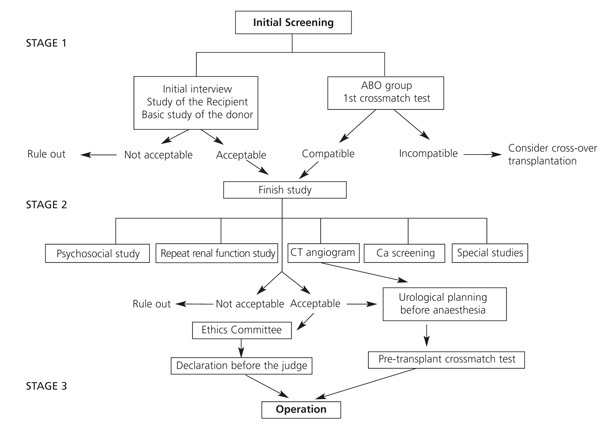
We have put forward a possible outline for most situations in Tables 1-3, and in Figure 1:
First Stage
The donor-recipient pair undergoes initial screening with basic non-invasive examinations, which can be performed immediately to find out if the donation is possible. The aim of this is to rule out any unfeasible cases causing as little inconvenience as possible to the donor and using as few resources as possible. It also gives the donors some time to confirm their decision.
It includes two fundamental aspects:
1. Review the recipient’s pre-transplant study. Confirm the indication for kidney transplantation, appropriate and comprehensive pre-transplant study, and risk and prognosis assessment. Another article of these Guidelines goes into this in great detail.
2. Donor-recipient compatibility. Ascertain or confirm the ABO group. HLA typing of the donor (and recipient, if not included in the deceased-donor waiting list) and first crossmatch test. This paves the way for the possibility of a direct LDKT if they are ABO compatible and have a negative crossmatch. If not, a crossover LDKT can be offered (see article “Immunological study of the donor-recipient pair” in this issue.)
Informed consent for the LDKT may be obtained during this stage (in some hospitals this is obtained during the first meeting) or later on, after the donor has had some time to take in all the information. However, this must be obtained before performing any test that is invasive or poses any risk.
Second Stage
Finish the studies. Any hospital that has special patient support must obtain specific reports from a psychologist and/or social worker to confirm the donor’s willingness and the circumstances surrounding the donation.
A second test must be performed to confirm that the kidneys are functioning normally and the donor must undergo suitable tests to rule out cancer. Imaging test should be carried out at the same time to study the vessels and urinary tract to confirm whether LDKT is viable and to plan the procedure. Other specialist doctors should be consulted or special tests should be performed during this stage (or during the previous one if there is an obvious problem), depending on the case. We will review this issue below.
Third stage
Immediately before the transplant. The crossmatch test is repeated. Legal procedures by the Ethics Committee to approve the transplantation. Under Spanish law, donors must provide a declaration of intention to donate before a civil registry judge.
STUDIES AND EXAMINATIONS
Next, we will go through the recommended studies and how they should be interpreted.
Medical history
Personal details: age, profession, family situation.
Family history, with special attention to: kidney diseases, HBP, diabetes, lupus, premature death from heart disease and cancer.
Personal medical history and current clinical situation, with special attention to: a) allergies; b) habits: tobacco, alcohol, drugs; c) surgical operations; pregnancies; thrombosis; d) nephro-/uropathies: lithiasis, urinary tract infections, haematuria, oedemas; e) cardiovascular: risk factors HBP, diabetes, dyslipidaemia, etc.; f) risk of infection: previous or current infections, transfusions, holidays or living abroad, unprotected sex, tattoos, imprisonment; g) cancer; h) mental or emotional disorders, addictions, and i) current medication. Use of non-steroidal anti-inflammatory drugs and herbal medicines.
Age
For legal reasons the minimum age for donation is 18 years in Spain. It is particularly important to assess if very young donors are mature enough to make their own decisions. There is no set maximum age limit (although donation is rarely considered in patients over 70 years old in Spain). In fact, the progressive ageing of the population on dialysis means that possible donors (for example, siblings or partners) tend to be elderly as well. These cases should be assessed in more detail; however, it is possible to be less strict on factors that depend on very long exposures, such as mild high blood pressure.
Habits
Tobacco smoking and excessive alcohol consumption (>60g/day) may justify the need for further tests and increase the risk of postsurgical complications in general. It is strongly recommended that these habits are stopped completely at least 4 weeks before the operation. It must be stressed that these habits must be stopped definitively, given that smoking increases the donor’s risk of death in the long term.18 Drug addiction is a contraindication to donation.
Physical examination
Complete physical examination, which includes taking the pulse in the lower limbs and a detailed skin examination.
Repeated blood pressure measurements (on at least three separate visits) using the technique recommended by experts (Spanish Society of Hypertension22). Ambulatory blood pressure monitoring (ABPM) is recommended when values are on the upper/lower limits, there is suspected white-coat hypertension and especially in patients over the age of 50.
Weight and height: body mass index (BMI) and waistline.
High Blood Pressure
Blood pressure must be <140/90mm Hg on separate visits; average measurements in ABPM must be <135/85mm Hg during the day and <120/75 mm Hg during sleep.
Someone with mild or moderate high blood pressure who has no other cardiovascular risks and has good renal function can donate as long as certain conditions are fulfilled: a) they are over 50 years old; b) they are not Afro-American; c) no evidence of damage to the internal organs as a result of HBP (ECG electrocardiogram, ophthalmoscopy, microalbuminuria <30mg/day); d) blood pressure can be controlled by lifestyle changes and the use of no more than one anti-hypertension drug, and e) there is a reasonable guarantee that the donor will follow the check-up period and treatment indefinitely.
As occurs in the general population, HBP is associated with a higher donor mortality rate in the medium term.18 Furthermore, and although data is conflicting, a recent meta-analysis seems to confirm that donating is associated with a 5mm Hg increase in blood pressure.20 However, in the general non-diabetic population the risk of CKD secondary to mild HBP, or secondary to increases in blood pressure such as the one cited above, is low and very long-term.23 Furthermore, with a suitable selection process, HBP donors do not seem to have worse renal function after donating if their blood pressure is managed correctly, at least in the short term,24 All the above would justify using elderly donors with mild HBP if a suitable follow-up is expected.
Obesity
Severe obesity (BMI>35) is a contraindication to donation, as it is associated with a greater surgical risk and greater risk of developing CKD in the long term. Obesity (BMI=30-35 or waistline >82cm in women or >102cm in men) may also be a contraindication if it is associated with other risk factors such as HBP, abnormal baseline glycaemia or family history of this condition and microalbuminuria. In any case, donors must be informed about the possible higher long-term risk and they must be given guidance so that they can lose as much weight as possible before the operation and then maintain their weight indefinitely afterwards by making changes to their eating habits. In spite of the above, some recent studies have found good short-term results in obese patients from a surgical25 and general point of view.26
Kidney assessment
Tests
Renal function measurement. The glomerular filtration rate (GFR) has traditionally been considered the best overall marker for renal function.
Plasma creatinine level. Of limited value, although it may be useful as a pre-selection criterion if it is known beforehand: donation may be ruled out with a confirmed value equal to or above 1.5mg/dl.
Creatinine clearance in a 24-hour urine sample. A 24-hour urine sample must be collected. The patient must not have a fever, menstruation, urinary infections or have undergone prior strenuous physical exercise. The possibility of an incomplete or excessive urine sample must be assessed by measuring the creatinine clearance (it must be within the range of 15-25mg/kg). This must be performed on at least two occasions to minimise any measurement errors. It is the basic procedure used by most hospitals given its general resources.
Use of Cockroft-Gault, aMDRD and CKD-EPI as complementary tests, given that these formulae are not validated for this specific population. However, the average creatinine clearance and MDRD have been reported to provide a good estimate of the GFR using the 125I iothalamate.27 The recent CKD-EPI formula may provide a more accurate GFR measurement than other formulae in patients with normal renal function.28
Direct measurement of GFR using exogenous non-isotopic markers (insulin, iohexol, iothalamate) or using isotopic techniques (125I iothalamate, 51Cr EDTA, 99Tc DTPA), depending on the resources of each hospital. This has generally been advised11,29 because the estimated GFR using formulae or the creatinine clearance with a real GFR measurement are not very accurate (correlation and error percentage).29 Therefore, if the hospital has a standardised technique, it should be used for the direct measurement of GFR. However, this would only be required when creatinine clearance levels are near limit values. The study with 99Tc DTPA has the added advantage of estimating the function of both kidneys separately. This is important if a marked difference in the size of the kidneys has been found in the imaging tests.30
Kidney volume estimated using imaging techniques. It has been proposed that this could also be helpful when estimating renal function,31,32 although for the moment guidelines are not sufficiently validated.
Measurement of 24-hour proteinuria and microalbuminuria (the measurement of protein/creatinine ratios in isolated samples is not acceptable11) and urinary sediment. See above for possible sources of error when collecting samples.
Abdominal-Doppler ultrasound, with special attention to the kidneys (size, structure, lithiasis, arterial blood flow) and the pelvis.
CT angiogram or MR angiogram with three-dimensional reconstruction and excretory phase study. This provides an anatomical assessment of the arterial vascularisation (identification of the main artery, accessory and/or aberrant arteries or early divisions), of venous vascularisation (number and situation of the veins, main vein, size and anastomotic abnormalities), of the kidney parenchyma and variations in the collecting duct system. At the same time, the rest of the abdomen can be examined to look for any possible neoplasia. The hospital would decide which of the two techniques to perform, given that both are not invasive, have good results, allow for three-dimensional reconstruction, have little intra-observer variability and high sensitivity and specificity in identifying the vasculature.33,34 The CT angiogram, compared to the MR angiogram, measures exposure to iodine contrast and radiation, is quicker, detects calcifications and defines the venous anatomy better. It is therefore considered the method of choice3-5 and is currently the most used procedure in Spain.
Selective kidney arteriography. Direct arterial study need only be used in cases with suspected stenosis or fibromuscular dysplasia due to the high reliability of the previous techniques.35
Renal function
This is a critical point in the assessment of the potential donor given the decisive influence this has on how the graft and the donor’s remaining kidney will function. Donors lose 50% of their GFR after the nephrectomy but this is recovered quickly, almost entirely during the first week. After a year the remaining kidney can make up for between 20%-40% of the initial renal function. This is influenced by age, sex, race and body size, although the main determining factor of the final GFR is the renal function level before the nephrectomy.29,36
The Amsterdam Consensus established as a general rule that a creatinine clearance <80ml/min/1.73m2 rules out the possibility of donation.11 However, this does not take into account that the measured renal function is lower in women and decreases throughout life.36 Therefore, 2 standard deviations below the normal limit has also been proposed as a lower limit for age, sex and body size corrected by 1.73m2. Thus, a clearance >90ml/min/1.73m2 is desirable in patients under 40 years old,3 especially in obese subjects.37 While, lower clearances (around 70ml/min and even lower) are accepted in many hospitals in elderly donor (>60 years old). The British guidelines3 propose that the lowest acceptable renal function depends on the age of the donor, based on the analysis of a sample of 428 donors. Although, it admits that there is not enough evidence available on donors’ actual evolution, especially when they are over 60 years old. Taking into account that after donation the GFR recovers up to 70% of its pre-donation level and that from 40 years old the kidneys lose renal function at a rate of 0.9ml/min/1.73m2 per year, the minimum acceptable GFR to donate would be the GFR that meant the donor would reach 80 years old with a GFR of at least 37.5ml/min/1.73m2. Minimum values calculated in ml/min/1.73m2: donors up to 40 years old 86; 50 years old 77; 60 years old 68; 70 years old 59.
If there is not much difference in the function of the two kidneys (less than 5%), the kidney to be extracted can be chosen according to anatomical preference. If the difference in kidney function is higher, the kidney chosen to be extracted should be the one with the lowest renal function.30 In fact, the donation may be ruled out if there is a very large difference in the functioning of the two kidneys (more than 10%), as it cannot be guaranteed that the donor’s or recipient’s renal function will be adequate.
Proteinuria
A proteinuria >300mg/day rules out donation.
The microalbuminuria value is not clearly defined for donation: a microalbuminuria >30mg/day is a relative contraindication. Donation is normally advised against when donors have extreme values of proteinuria (150-300mg/day) or microalbuminuria (30-300mg/day). However, each case can be evaluated individually, taking into account other factors such as age, obesity, HBP or glucose metabolism abnormalities.
Haematuria
A microhaematuria (>3 red blood cells/ field or 5 red blood cells x106/l) means that lithiasis or microlithiasis must be studied (as can be seen below), and urinary cancer must be ruled out by an extensive urological study (cytology, imaging tests or cystoscopy if needed).
A kidney biopsy will be needed if there is a possibility that the haematuria is caused by a glomerular disorder (dysmorphic red blood cells)38 glomerulopathies (IgA/IgM nephropathy, Alport’s syndrome, thin membrane), medullary sponge kidney and significant glomerulosclerosis.
Leukocyturia
It must be persistent when there is no urinary tract-prostate infection.
Urinary TBC, a contraindication to donation, must be ruled out by mycobacterial culture (at least three tests).
If it cannot be explained by an infection, a kidney biopsy may be needed to rule out interstitial nephritis or chronic pyelonephritis (which would also rule out donation).
Abnormalities in imaging tests
Structural abnormalities that would rule out donation may be detected: a) large differences in the size of the two kidneys or significant unilateral atrophy; horseshoe kidney; b) extensive cortical scarring; c) more than 2-3 cysts in both kidneys, or complex or multilocular cysts (see below); d) angiomyolipoma, tumours in general; e) significant arteriosclerosis; f) fibromuscular dysplasia; g) multiple or large lithiasis (see below); h) dilation or obstruction of ducts, and i) medullary sponge kidney.
The presence of multiple arteries (up to three) or multiple veins or certain urinary tract abnormalities (for example, duplicity) is not strictly a contraindication to donation, but it may have an effect on which kidney should be extracted and the technique used, which would be based on the surgical team’s criteria.
The article “Surgical aspects of living-donor kidney transplantation” in this issue will look at these questions in more detail.
Special situations
Lithiasis39,40
Study: If a patient has a history of lithiasis, it is necessary to assess the timeline and make-up of the calculi, current lithiasis (using imaging tests), and carry out a metabolic study.
Renal lithiasis is an absolute contraindication in many cases: a) nephrocalcinosis; b) bilateral renal lithiasis; c) current unilateral lithiasis with calculi >1.5cm, and d) recurring lithiasis or possible recurring lithiasis due to an associated disorder: hypercalciuria, hyperphosphataemia, hypocitraturia, hyperuricaemia-uria, cystinuria, hyperoxaluria, distal tubular acidosis, recurring urinary tract infections, sarcoidosis, inflammatory bowel disease.
Donation would be allowed in the case of a single episode of urolithiasis which occurred a long time ago (>10 years). A kidney with lithiasis may be used if the calculus (or calculi, up to 2-3) is small and can be removed. However, if the donor is currently suffering from lithiasis or has recently had calculi removed, there is a high probability that they will recur (up to 50% during the first 5-7 years)1 and this risk must be debated and taken into consideration, especially in young donors. In any case, donors must be warned about the need to watch out for lithiasis for the rest of their lives.
Cysts and polycystic disease
If there is no family history of polycystic disease (PC), the presence of a small, isolated cyst (<1cm) is not an obstacle for donation. Larger isolated simple cysts can also be allowed (up to 5cm, Bosniak category 1), although the surgeon may decide to perform a kidney biopsy with excision and closure.41,42 Some hospitals also take out organs from donors over the age of 40 years with multiple cysts (up to four), with no apparent problems in the medium term.41,42
It is necessary to rule out that donors with family history of polycystic diseases are asymptomatic carriers of the disease. The main method is an ultrasound test using the diagnostic criteria established for PC secondary to a mutation in the PK1 gene (85% in the general population)43:
1. Up to 29 years old: at least 2 cysts (unilateral or bilateral).
2. 30-59 years old: at least 2 cysts in each kidney.
3. 60 years old or more: four or more cysts in each kidney.
There is a 100% positive and negative predictive value over the age of 30 years old. If it is conclusive, the decision can, therefore, be made solely with this test. Over 30 years old, the negative predictive value does not reach 100% (4% false negatives).
These rules are also applied in PC linked to a PK2 mutation with regards a positive diagnosis, but the negative predictive value is not well defined. It is, therefore, not possible to rule out the disease completely.
Thus, the dilemma occurs in certain cases: uncertain findings in the ultrasound, patients under 30 years old or families with undefined disease or disease linked to PK2 gene that do not meet all the ultrasound diagnostic criteria and cases of multiple cysts in individuals with no family history (possible PC due to mutation de novo or mutation linked to PK2 in small families with few clinical symptoms, where, as a preliminary step, an ultrasound test should be performed on the whole family).
In these cases, other resources may be used:
- Study with CT scan and, above all, with nuclear magnetic resonance imaging,44 as it has greater sensitivity and can detect smaller cysts, but its results are not completely validated. It helps to rule out the disease in young donors, but on the other hand, it can give false positives.
- Direct genetic study of the donor and recipient.45 This is useful to rule out donation if the result is positive, but in more than 30% of cases it fails to detect the mutation.
- Genetic linkage analysis,46 where different family members need to be studied, whether they are ill or not.
Other hereditary diseases
Renal function of family members must be studied in great depth when the recipient has hereditary diseases, including a biopsy in certain cases:
1. Alport’s Syndrome: hearing and eye tests. Donation can be considered in men >20 years old and women without haematuria, although the risk of developing kidney damage cannot be completely ruled out without performing a genetic study or electron microscopy.
2. Thin basement membrane: donation can be considered in donors over 40 years old that do not have HBP or proteinuria.
3. Glomerulopathies found in family members (IgA, focal and segmental, membranous, membranoproliferative). If there is familial clustering of a kidney disease, it must be suspected.
4. Lupus: the study of the donor will include autoantibodies, complement and antiphospholipids.
5. Atypical haemolytic-uraemic syndrome: genetic study to determine the risk of relapsing and the risk of developing the disease later in the donor.
Cardiovascular assessment
The aim of this assessment is to rule out any significant heart diseases that are a contraindication to donation due to the higher risk for the donor: ischaemic heart disease, heart failure, valvular heart disease, significant left ventricular hypertrophy or significant arrhythmia. When the donors go through an appropriate selection process, they do not seem to be at a higher cardiovascular risk after the transplantation.47
The study must include at least auscultation, ECG and chest x-ray. Special tests and/or a medical visit with a cardiologist may be indicated depending on the risk profile and the preliminary exam of the donor.
1. Echocardiography (hypertensive patients, murmurs, dyspnoea on exertion, elderly patients).
2. Holter (suspected arrhythmia).
3. Cardiac stress tests (abnormal ECG, patient over 60 years old or combination of risk factors: age >45 years old in men, >55 years old in women, tobacco smoking, dyslipaemia, HBP, family history).
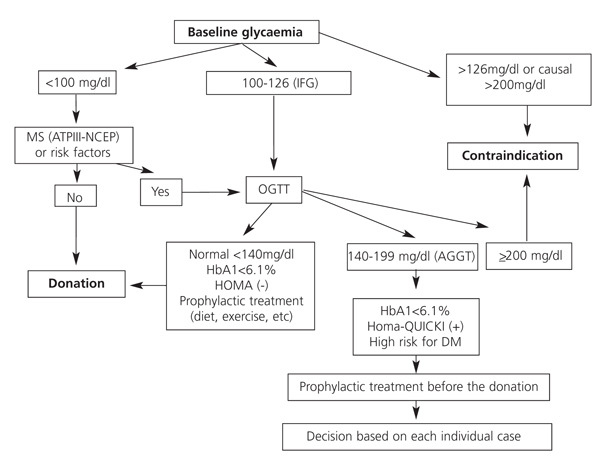
Diabetes and metabolic syndrome23,48
Minimum study: baseline glycaemia, HbA1c and lipid profile.
Indication to perform functional tests, essentially the oral glucose tolerance test (OGTT): a) first-degree family history of type 2 diabetes; b) abnormal baseline glycaemia (100-125mg/dl) or HbA1c>6%-6.5%; c) obesity, and d) other data that increase or create risk for metabolic syndrome: high blood pressure, dyslipaemia (triglycerides >150mg/dl or HDL cholesterol <35 in men/<39 in women), microalbuminuria.
For donation:
1. Previous history or diagnosis of diabetes (baseline glycaemia >126 on two occasions, or non-fasting glycaemia or two hours after the OGTT>200) is an absolute contraindication to donation.
2. Previous history of gestational diabetes is an absolute contraindication to donation given the high rate of developing diabetes later in life.
3. Abnormal baseline glycaemia and hydrocarbon intolerance (glycaemia between 140 and 199 after 2 hours) are a relative contraindication to donation and must be assessed individually, taking into account the response to a simple health plan (diet, exercise, statins).
We would be inclined to rule out donation when abnormal baseline glycaemia is in the upper range (110-125), or there is family history of the disease, other risk factors or metabolic syndrome as these show a higher tendency to develop diabetes and kidney disorders later in life. Hernández proposes an all-round approach to the problem that may be very useful (Figure 2).48
Respiratory
Lung function tests would be indicated in heavy smokers and when there are symptoms which would point to chronic lung disease. There is an increased risk for donors when forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) is <70%, or FEV1/FVC ratio is <65%.
Cancer detection
Study
Colon: indicated according to the recommendations for the general population ( first-grade family history of the disease, age >50 years old and others). Minimum: faecal occult blood test. Colonoscopy is recommended.
Breast: Mammography/ultrasound for women >40 years old, or if they have a family history of breast cancer.
Uterus: cervical cytology and pelvic ultrasound.
Prostate: rectal examination and prostate-specific antigen for men >50 years old, or if there is family history of early prostate cancer.
Specific studies according to the findings of the preliminary study or the donor’s previous or family history; for example: dermatology exam if there is family history of melanoma or a high number of naevus.
Donation is ruled out if there is a previous diagnosis of haematological, gastrointestinal, testicular, melanoma, lung, breast, kidney or urinary cancers, choriocarcinoma or monoclonal gammopathy.
Donation may be considered in selected cases when the cancer is considered curable and when there is no risk of transmission, after discussing it with the donor-recipient pair. For example: non-melanoma skin cancer, cancer in situ or incidental tumours (cervix, colon). In any case, the previous treatment of the neoplasia should not have decreased the kidney reserve or lead to a greater surgical risk.
Detecting infections
Table 3 summarises the studies carried out to stop infections being transmitted to the recipients, including those which are dependant on where the donor comes from.49,50 Some tests must be repeated if the infections were discovered a long time ago, or if any indicative signs or symptoms appear, especially periodic fever syndrome. Certain tests must be performed on donors at risk to make sure they are negative (HBV, HCV, HIV, through serological and viral load testing). These must be performed just before the donation to cover for the window period of a recent infection. Certain active or latent infections will not be contraindications if the donor can be treated effectively.
Tuberculosis
If the PPD is positive, active TBC must be ruled out (this is a contraindication) based on symptoms, imaging tests (chest CT-x-ray, intravenous pyelogram) and microbiological tests (mycobacterial cultures in sputum and urine). Latent TBC is not a contraindication. Treating it before donation is recommended (9 months of isoniazid or 3 months of rifampicin), although this has not been found to be beneficial in high endemic regions.51 Considering a post-transplant prophylaxis in the recipient is also recommended in this case.
Syphilis
If the RPR test is positive, this must be followed up by treponemal tests. The presence of latent syphilis is not a contraindication, but the donor must receive appropriate treatment (3 weekly doses of 2.4 million units of penicillin-benzathine i.m., II-3).
Herpes virus
If a donor is found to be positive and the recipient negative for CMV or Epstein-Barr virus, then there is a high risk of transmission. A strict follow-up must be implemented and the recipient must be treated for CMV with prophylaxis or advance treatment. In both cases, if the IgM antibody is positive, this may be indicative of a recent infection: the viral replication should be controlled with PCR and the donation should be postponed until it comes back negative.
It has not been well established whether it is necessary to screen for herpes virus 6 and 7 (found almost universally) and herpes virus 8 (prevalence varies a lot depending on the region).
Hepatitis and HIV
Positive results for HbsAg or presence of viral DNA in the blood is a contraindication to donation in HbsAg-negative patients. Positive result for HBcAb-IgM, which indicates a recent infection, and isolated positive results for HBcAb mean that active replication by viral DNA and mutants must be ruled out. Positive results for HbcAb-IgG with/without positive HbsAb and with negative viral DNA mean that there is a very small risk of transmission (although not completely risk free), making donation possible. However, the recipient should be immunised naturally or by effective vaccination. Transplantation from an HBcAb-positive donor to a non-immunised patient should only be performed after the pair is given detailed information. The patient must be protected using specific immunoglobulin and/or lamivudine.52
A positive result for HVC or HIV is a contraindication to donation.
Other
Donors from or who have lived in endemic areas for other infections (see Table 3) should be tested for these infections, preferably in consultation with the infectious diseases department. Donation can be considered in cases of diseases such as Chagas disease, bilharziasis or anguilluliasis after they have been treated effectively.
Urinary tract infections
Lower urinary tract infections in women, even if they are recurrent, are not a contraindication to donation. However, unexplained recurrent pyelonephritis is a contraindication. Anatomical abnormalities must be ruled out when the patient has persistent infections or previous history of recurrent infections from childhood. Imaging tests should be used (CT scan, intravenous pyelogram, voiding cystourethrography) and even cystoscopy.
In any case, the urine must be sterile at the time of the donation.
Other
Perform a pregnancy test on women of child-bearing age.
Coagulation blood test: some hospitals perform this test systematically, although it is generally only indicated if there is previous history of thrombosis. Previous history of pulmonary thromboembolism or deep vein thrombosis may be a contraindication to donation. Women who are carriers of the factor V-Leiden mutation are advised to stop taking any anti-contraceptive drugs or hormone replacement treatment before surgery.53
Psychosocial aspects
Donors must visit a psychiatrist or a clinical psychologist if there is a chance that they suffer from any mental disorders or intellectual deficiencies. Although, a psychiatric diagnosis does not necessarily rule out donation.54 Hospitals vary greatly in this respect,55 but the systematic study of psychosocial aspects in all donor-recipient pairs by a psychiatrist or clinical psychologist and a social worker with experience in LDKT is very important.
Psychological study
The donor’s ability to consent is assessed by looking at the donor’s life history and performing a complete psychological and psychopathological assessment. It is important to assess how much the donor knows about the transplant, quality of life with one kidney, benefits for the recipient, risks and the chance of failure. Other aspects that must be assessed are: whether the donor is internally or externally being coerced or pressurised into the decision, and the donor’s expectations for the results, identifying if there are any discrepancies between these and reality. If there is, the donor must be correctly informed about the expected results before the LDKT.
Social study
The following aspects need to be assessed:
1. Donor’s and recipient’s situation: personal autonomy and social life of both of them; economic and employment situation.
2. Family situation: family dynamics and organisation, relationship between members, contradictions and influence, ability to solve conflicts. Housing conditions.
3. Legal situation and support networks.
After the assessment an interventionplan is put in place for any areas where deficiencies were found, discussing it with the donor, recipient and their families. This includes any measures during the hospitalisation or immediate postoperative period, identifying any support that is needed in the hospital and at home. The aim of this plan is to strengthen any protective factors and training people so that they can solve their own problems, using family mediation techniques.
ACKNOWLEDGEMENTS
Asunción Luque, Agustina Hervás and Virginia Cabello for their contribution to this study.
Table 1. General outline of the donor study
Table 2. Minimum laboratory tests
Figure 2. Intervention algorithm to detect diabetes mellitus before donation
Figure 1.
10691_108_14240_en_10691_tabla_3.doc
Table 3. Detecting infections