Hypomagnesaemia in haemodialysis (HD) is associated with increased mortality risk: its relationship with dialysis fluid (DF).
IntroductionLow concentrations of magnesium (Mg) in blood have been linked to the development of diabetes, hypertension, arrhythmias, vascular calcifications and an increased risk of death in the general population and in haemodialysis patients. The composition of the dialysis fluid in terms of its magnesium concentration is one of the main determinants of magnesium in haemodialysis patients.
ObjectiveTo study magnesium concentrations in haemodialysis patients, their predictive mortality rate and what factors are associated with hypomagnesaemia and mortality in haemodialysis.
MethodsRetrospective study of a cohort of prevalent haemodialysis patients followed up for two years. Serum magnesium was measured every six months. The analysis used the initial and average magnesium values for each patient, comparing patients with magnesium below the mean (2.1 mg/dl) with those with magnesium above the mean. During the follow-up, three types of dialysis fluid were used: type 1, magnesium 0.5 mmol/l; type 3, magnesium 0.37 mmol/l (both with acetate); and type 2, magnesium 0.5 mmol/l with citrate.
ResultsWe included 137 haemodialysis patients in the study, of which 72 were male and 65 were female, with a mean age of 67 (15) [26–95] years old. Of this group, 57 patients were diabetic, 70 were on online haemodiafiltration (OL-HDF) and 67 were on high-flow haemodialysis (HF-HD). The mean magnesium of the 93 patients with dialysis fluid type 1 was 2.18 (0.37) mg/dl. In the 27 patients with dialysis fluid type 3 it was 2.02 (0.42) mg/dl. And in the 17 with dialysis fluid type 2 it was 1.84 (0.24) mg/dl (p = 0.01). There was a pronounced direct relationship between Mg and P and albumin. After a mean follow-up of 16.6 (8.9) [3–24] months, 77 remained active, 24 had died and 36 had been transplanted or transferred. Patients with magnesium above than 2.1 mg/dl had a longer survival (p = 0.008). The survival of patients with the three types of dialysis fluid did not differ significantly (Log-Rank, p = 0.424). Corrected for blood magnesium, patients with dialysis fluid with citrate have better survival (p = 0.009). The COX regression analysis shows how age, serum albumin, magnesium, dialysis technique and type of dialysis fluid have an independent predictive mortality rate.
ConclusionsLow serum magnesium levels have a greater association with an increased risk of mortality compared to high levels. The type of dialysis fluid affects the magnesium concentration and the risk of death.
La hipomagnesemia en hemodiálisis (HD) se asocia a mayor riesgo de mortalidad: su relación con el líquido de diálisis (LD).
IntroducciónConcentraciones bajas de magnesio (Mg) en sangre se han relacionado con el desarrollo de diabetes, hipertensión arterial, arritmias, calcificaciones vasculares y con mayor riesgo de muerte, en población general y en hemodiálisis. La composición del LD y su concentración de Mg es uno de los principales determinantes de la magnesemia en los pacientes en HD.
ObjetivoEstudiar las concentraciones de magnesio en los pacientes en HD, su valor predictivo de mortalidad y qué factores se asocian a la hipomagnesemia y mortalidad en HD.
MétodosEstudio retrospectivo de una cohorte de pacientes prevalentes en HD seguidos 2 años. Cada 6 meses se determina el Mg sérico. En el análisis se utiliza el Mg inicial y el medio de cada paciente, comparando los pacientes con Mg por debajo de la media, 2,1 mg/dl, con los que están por encima. Durante el seguimiento se han utilizado 3 tipos de LD: tipo 1, magnesio de 0,5 mmol/l y tipo 3, Mg 0,37 mmol/l ambos con acetato y tipo 2, 0,5 mmol/l de Mg con citrato.
ResultadosSe han incluido en el estudio 137 pacientes en hemodiálisis, 72 hombres y 65 mujeres, con una edad media de 67(15) [26–95] años. 57 pacientes eran diabéticos y 70 pacientes estaban en hemodiafiltración en línea (HDF-OL) y 67 en hemodiálisis de alto flujo (HD-HF). El Mg medio de los 93 pacientes con LD tipo 1 era: 2,18(0,37)mg/dl, en 27 con el tipo 3: 2,02 (0,42)mg/dl y los 17 con tipo 2: 1,84 (0,24)mg/dl (p = 0,01). El Mg se relaciona de forma directa significativa con el P y con la albumina. Después de un seguimiento medio de 16,6(8,9)[3–24] meses, 77 seguían activos, 24 habían fallecido y 36 se habían trasplantado o trasladado. Los pacientes con un Mg superior a 2,1 mg/dl tienen una supervivencia mayor, p = 0,008. La supervivencia de los pacientes con los tres tipos de LD no difería significativamente, Log-Rank, p = 0,424. Corregido por la magnesemia, los pacientes con LD con citrato tienen mejor supervivencia, p = 0,009. En el análisis de regresión de COX se observa como la edad, albumina sérica, Mg, técnica de diálisis y tipo de LD tienen valor predictivo de mortalidad independiente.
ConclusionesLos magnesios séricos bajos respecto a los altos se asocian a mayor riesgo de mortalidad. El tipo de LD influye en la concentración de Mg y en el riesgo de muerte.
Low magnesium (Mg) concentrations in blood have been associated to the development of diabetes, high blood pressure, cardiac arrhythmias and even an increased risk of death in the general population1–8 and Mg intake is inversely related to the incidence of metabolic syndrome.9
Extracellular Mg correspond to 1% of the total body Mg. A 99% is contained in the intracellular compartment. Normal values of serum are 0.71–1.05 mmol/l (1.7–2.5 mg/dl), which are distributed as a 33% bound to proteins, 62% as ionized Mg and a 5% as anions complexes.10–12 Serum [Mg] levels is partially regulated, its functional reservoir is the bone.
The intake of Mg is associated with the intake of foods recommended in a healthy diet.11,12 One third of the Mg ingested would is absorbed.13 The net intestinal absorption of Mg is 100 mg/day, which is balanced by a renal excretion of 100 mg/day, after a tubular reabsorption of 2300 mg/day. In renal failure, serum Mg tends to increase due to lower filtration, although the tubular reabsorption is decreased.14
In hemodialysis (HD) patients, without significant residual renal function, Mg elimination takes place during the dialysis sessions. Therefore, the serum Mg level will depend on the intake, which is generally decreased in the recommended diets11,12 and the dialysance of Mg. The dialysance of Mg depends on the effectiveness of the HD procedure, the K and the concentration of Mg in the dialysate. Generally, dialysis is performed with a Mg concentration of 0.5 mmol/l (1.2 mg/dl), so that the magnesium level is usually maintained around 2.3 and 2.4 mg/dl, but with that Mg concentration maintained in the dialysis fluid there are patients with hypomagnesemia and also with hypermagnesemia. It is also necessary to take into account the use of medication containing Mg in the composition15 or those that interfere with Mg absorption such as omeprazole.12
In HD patients, serum Mg concentrations are associated with mortality.16–22 Cardiac arrhythmias are a very prevalent cause of death in HD patients. Hypomagnesemia is associated with increased QT and arrhythmias23; the simultaneous occurrence of changes in serum potassium and metabolic alkalosis may potentiate the arrhythmogenic effects of hypomagnesemia. Cardiovascular mortality is the most prevalent in HD patients. Vascular injury and calcifications are frequent in HD patients and are associated with a high risk of death. The magnitude of vascular calcifications in HD patients is inversely related to magnesium levels.24 It has been shown that Mg supplementation reduces the thickening of the arterial wall.25
To interpret correctly the association between hypomagnesemia and mortality, it is essential to know the cause of hypomagnesemia. One possibility is that hypomagnesemia is associated to a low Mg intake, malabsorption or malnutrition. Another possibility is a negative Mg balance during hemodialysis that is not compensated by Mg intake.
For years we have been using dialysate with citrate instead of acetate as a stabilizer. For the same calcium concentration in the dialysate, the calcium balance in HD is lower with citrate than with acetate.26–29 This is due to the increase in calcium associated with citrate that diffuses from the blood to the dialysis fluid. Something similar happens with Mg that could explain the decrease in magnesium levels with the use of citrate in the dialysis fluid.30
ObjectiveTo evaluate the Mg concentration in the HD patients. Investigate the predictive value of Mg concentration on mortality on hemodialysis patients and what are the factors associated with both hypomagnesemia and mortality.
- 1
To determine the magnesium concentrations in HD patients and its value as a predictor of mortality.
- 2
Analyze if patients with citrate in the dialysis fluid have lower levels of Mg than with acetate.
- 3
Study what factors are associated with hypomagnesemia and mortality in HD.
This is a retrospective study to assess survival in a cohort of prevalent HD patients from 2014, followed for 2 years until the end of 2016. Mortality during follow-up has been evaluated in relation to epidemiological parameters, comorbidities, hemodialysis procedure with special emphasis on the type of dialysis fluid and, laboratory parameters with special emphasis on Mg.
PatientsInclusion criteria: prevalent HD patients from the Infanta Leonor University Hospital Unit during 2014. Patients were on Chronic HD, over 18 years of age and signed an informed consent to allow the use of their clinical and laboratory information.
Exclusion criteria: patients with follow-up of less than 3 months or with less than 3 sessions of HD per week with significant residual renal function.
Hemodialysis techniqueThe dialyzers used wereAK200us® and Artis® (Baxter) and ST5008 (Fresenius, FMC, Bad Homburg, Germany). All machines have ionic dialysance (Diascan® or OCM®). The machines were connected to the TSS® computer application, automatically uploading the data for each session. Ultra-pure Dialysis fluid was used with levels of colony-forming units/ml below 0.1 and endotoxin levels below 0.03 EU/ml (SEN Guides). Patients were dialyzed 3 days per week for minimum of 12 hours a week, except those with significant residual renal function. There were high permeability dialyzers with synthetic membranes and a surface between 1.8 and 2.1 m2. Patients with adverse reactions to these membranes were dialyzed with cellulose triacetate. The blood flow was the maximum allowed by vascular access without a drop of arterial line pressure below
-220 mmHg. Dialysis techniques were: online hemodiafiltration (OL-HDF) and high-flow HD (HF-HD).
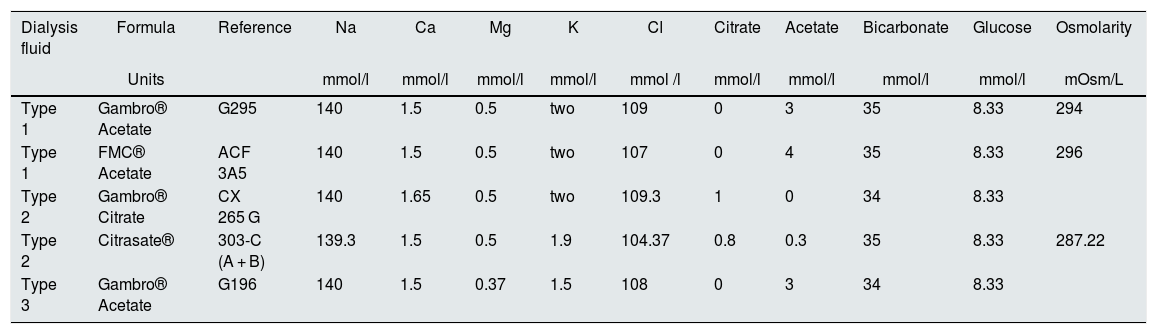
During follow-up, 5 formulas of dialysis fluid (LD) were used, which have been grouped into three types: type 1 (Mg of 0.5 mmol/ L) and type 3 (Mg 0.37 mmol/L) both with acetate and type 2 (Mg 0.5 mmol/L) with citrate. The full composition is specified in the Table 1.
Composition of the dialysis fluids used.
| Dialysis fluid | Formula | Reference | Na | Ca | Mg | K | Cl | Citrate | Acetate | Bicarbonate | Glucose | Osmolarity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | mmol/l | mmol/l | mmol/l | mmol/l | mmol /l | mmol/l | mmol/l | mmol/l | mmol/l | mOsm/L | ||
| Type 1 | Gambro® Acetate | G295 | 140 | 1.5 | 0.5 | two | 109 | 0 | 3 | 35 | 8.33 | 294 |
| Type 1 | FMC® Acetate | ACF 3A5 | 140 | 1.5 | 0.5 | two | 107 | 0 | 4 | 35 | 8.33 | 296 |
| Type 2 | Gambro® Citrate | CX 265 G | 140 | 1.65 | 0.5 | two | 109.3 | 1 | 0 | 34 | 8.33 | |
| Type 2 | Citrasate® | 303-C (A + B) | 139.3 | 1.5 | 0.5 | 1.9 | 104.37 | 0.8 | 0.3 | 35 | 8.33 | 287.22 |
| Type 3 | Gambro® Acetate | G196 | 140 | 1.5 | 0.37 | 1.5 | 108 | 0 | 3 | 34 | 8.33 |
The 5 formulas are grouped into 3 types of LD: type 1 (Mg of 0.5 mmol/L) and type 3 (Mg 0.37 mmol/L) both with acetate and type 2 (Mg 0.5 mmol/L) with citrate.
Ca: calcium; Cl: chloride; Glu: glucose; K: potassium; Mg: magnesium; Na: sodium.
The following parameters were analyzed: age, sex, underlying disease, comorbidity, dialysis technique HDF-OL/ HD-HF and Kt.
Biochemical parameters: sodium (Na), potassium (K), magnesium (Mg), total calcium (Ca t) and ionized Calcium (Ca++), phosphorus (P) and parathyroid hormone (PTH). The pH was measured by potentiometry, the pCO2 by Sveringhaus electrode, the pO2 by amperometry and the Ca++ by ion selective electrode (ISE). Biochemical determinations were obtained with an autoanalyzer (ADVIA® 2400 Chemistry System, Bayer). PTH determinations were made by chemiluminescence, using the ADVIA CENTAUR system from Bayer.
The serum concentration of Mg was was measured every 6 months. The initial Mg and the mean of the determinations in each patient were used for analysis.
Information was collected on whether or not the patient was on proton pump inhibitors (PPIs).
EvolutionThe patients were followed until the end of 2016. Information recorded included whether the patients was still active, had received a transplant, was lost to follow-up or died.
StatisticsVariables with normal distribution are expressed as the mean and the standard deviation. The only variable with a non-normal distribution was the C-reactive protein (CRP), Kolmogorov-Smirnov Z, and with Log transformation it was possible to transform it into normal distribution.
For statistical analyzes, the serum Mg concentration was considered the first predialysis serum Mg concentration determined in each patient upon inclusion in the study. The mean of the Mg determinations in each patient was also evaluated.
The variable predialysis serum Mg concentration has been categorized as low and high, using the mean value of the population, 2.1 mg/dl, as the cut-off point.
The differences of the analytical variables according to the types of dialysis fluid was analyzed by one-step analysis of variance after performing the Levene test.
Test of Pearson was used to analyze bivariate correlations between variables with normal distribution.
Kaplan–Meier survival curves have been calculated and the factors were compared using the log rank test (Mantel-Cox). Stepwise Cox regression has been used, to elucidate the variables that independently influence mortality.
A p < 0.05 has been considered significant. SPSS 15.0 (Chicago. Illinois. USA) has been used for statistical analysis.
ResultsThe study included 137 hemodialysis patients, 72 men and 65 women, with a mean age of 67(15) years, ranging from 26 to 95 years. The causes of CKD were: diabetes 32.8%, glomerulonephritis 16.1%; vascular disease 11.7%; tubulointerstitial nephropathy 7.3%; adult polycystic kidney disease 5.8%; other nephropathies 9.5% and a 16.8% of unknown etiology. At the time of the study, there were 57 patients with diabetes.
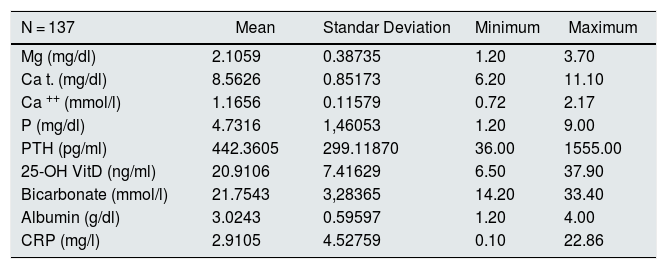
Table 2 provides the data on biochemical parameters at baseline. The mean initial Mg of the patients was 2.1 (0.39) mg/dl. Serum Mg was determined 578 times in the 137 patients, between 1 and 5 times per patient during follow-up. The overall mean Mg concentration was 2.07 (0.34) mg/dl, ranging among the patients from 1.2 and 3.75 mg / dl. In patients the initial Mg does not differ significantly from the average Mg.
Baseline biochemical data.
| N = 137 | Mean | Standar Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Mg (mg/dl) | 2.1059 | 0.38735 | 1.20 | 3.70 |
| Ca t. (mg/dl) | 8.5626 | 0.85173 | 6.20 | 11.10 |
| Ca ++ (mmol/l) | 1.1656 | 0.11579 | 0.72 | 2.17 |
| P (mg/dl) | 4.7316 | 1,46053 | 1.20 | 9.00 |
| PTH (pg/ml) | 442.3605 | 299.11870 | 36.00 | 1555.00 |
| 25-OH VitD (ng/ml) | 20.9106 | 7.41629 | 6.50 | 37.90 |
| Bicarbonate (mmol/l) | 21.7543 | 3,28365 | 14.20 | 33.40 |
| Albumin (g/dl) | 3.0243 | 0.59597 | 1.20 | 4.00 |
| CRP (mg/l) | 2.9105 | 4.52759 | 0.10 | 22.86 |
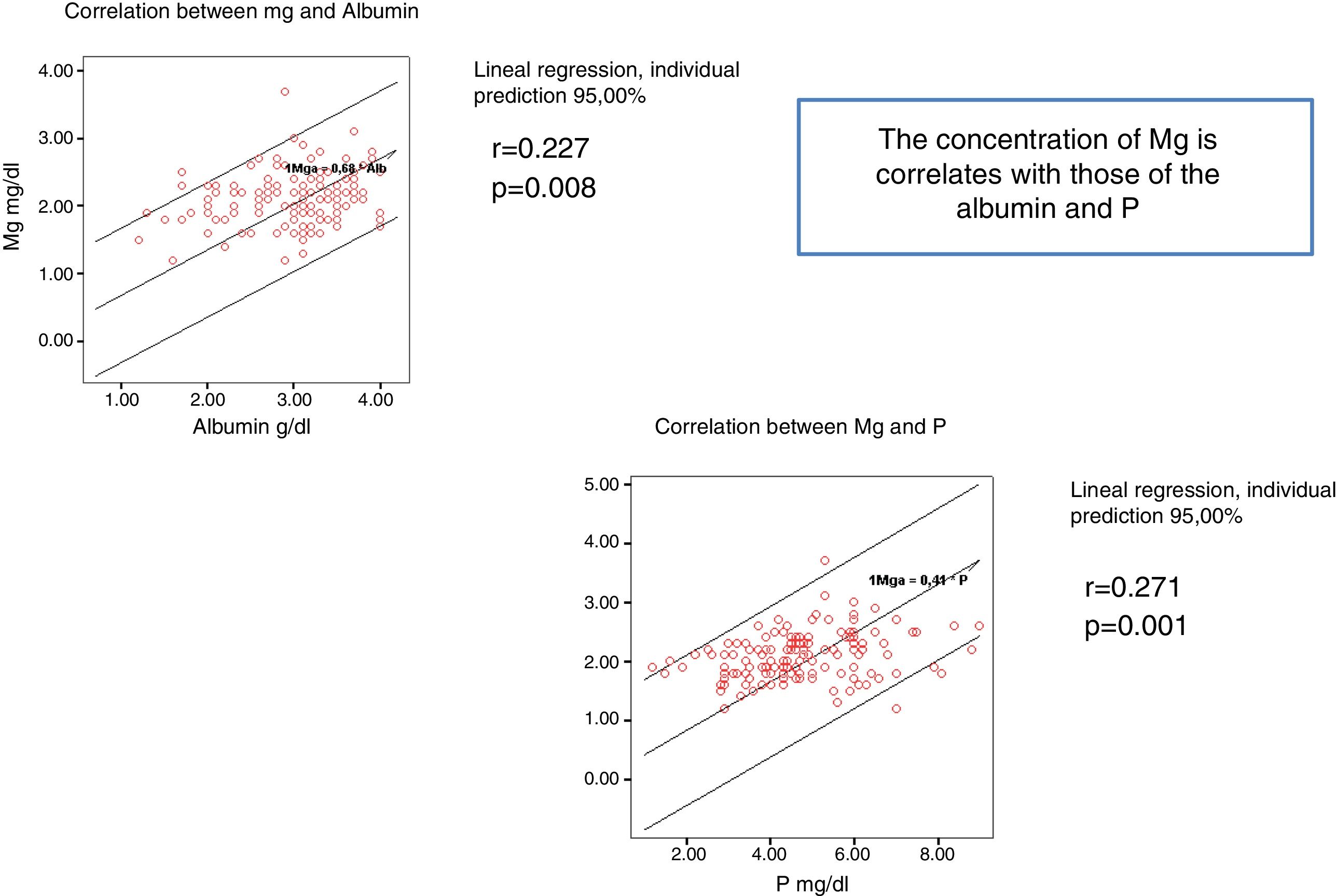
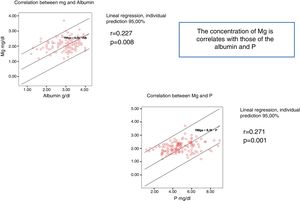
The serum Mg concentration significantly correlates with serum levels of P and Albumin (Fig. 1).
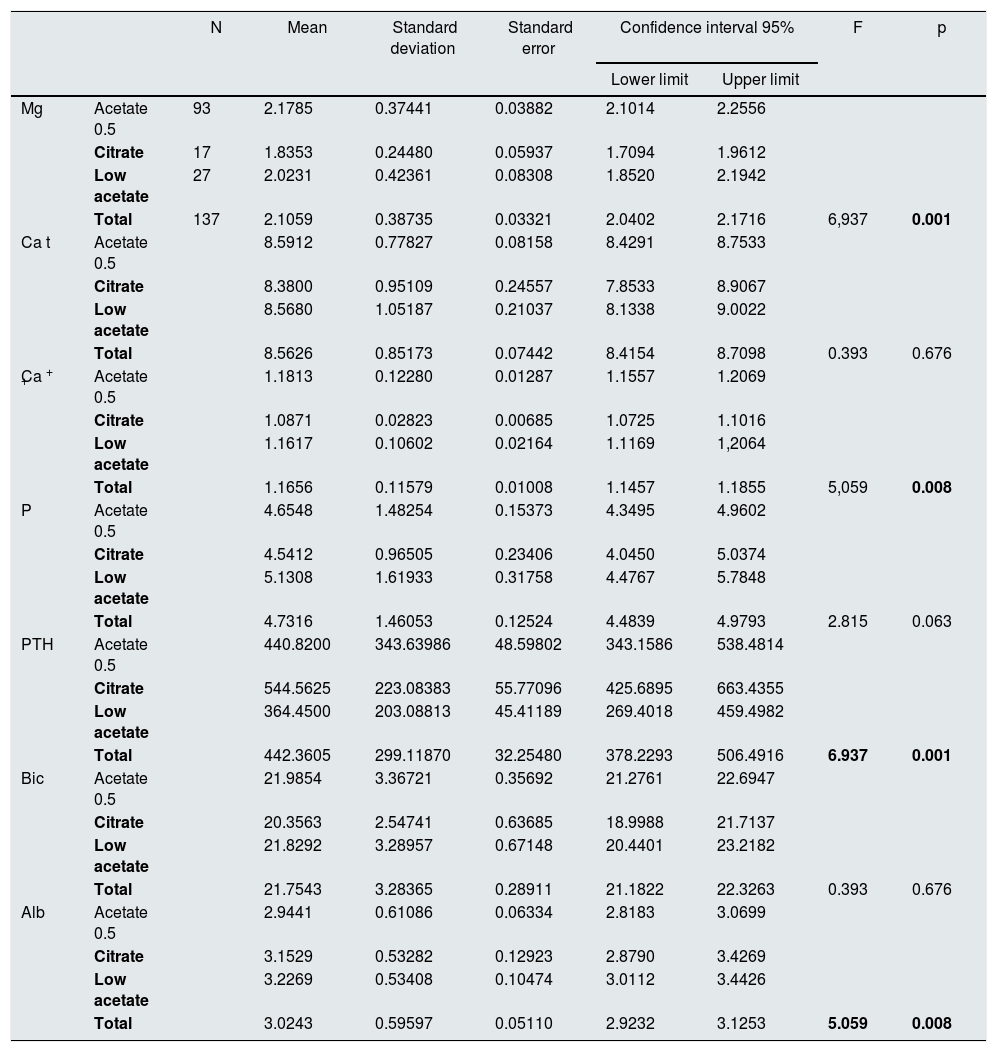
A 67.9% of the patients (n = 93), were dialyzed with a dialysis fluid (type 1) containing acetate and Mg 0.5 mmol/l; a 12.4% of patients (n = 17) were dialyzed, with dialysis fluid (type 2) containing citrate and Mg 0.5 mmol/l and a 19.7% (n = 27) were dialyzed with dialysis fluid (type 3) with acetate and Mg 0.37 mmol/l. The mean value of serum Mg concentration in the 93 patients dialyzed with type 1 of dialysis fluid was: 2.18 (0.37) mg/dl, in the 27 patients on type 3 the mean Mg was 2.02 (0.42) mg/dl and the 17 on type 2 the mean value of Mg was 1.84 (0.24) mg/dl (p = 0.01) (Table 3).
Pre-hemodialysis biochemical data separated according to the type of dialysis fluid.
| N | Mean | Standard deviation | Standard error | Confidence interval 95% | F | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||||
| Mg | Acetate 0.5 | 93 | 2.1785 | 0.37441 | 0.03882 | 2.1014 | 2.2556 | ||
| Citrate | 17 | 1.8353 | 0.24480 | 0.05937 | 1.7094 | 1.9612 | |||
| Low acetate | 27 | 2.0231 | 0.42361 | 0.08308 | 1.8520 | 2.1942 | |||
| Total | 137 | 2.1059 | 0.38735 | 0.03321 | 2.0402 | 2.1716 | 6,937 | 0.001 | |
| Ca t | Acetate 0.5 | 8.5912 | 0.77827 | 0.08158 | 8.4291 | 8.7533 | |||
| Citrate | 8.3800 | 0.95109 | 0.24557 | 7.8533 | 8.9067 | ||||
| Low acetate | 8.5680 | 1.05187 | 0.21037 | 8.1338 | 9.0022 | ||||
| Total | 8.5626 | 0.85173 | 0.07442 | 8.4154 | 8.7098 | 0.393 | 0.676 | ||
| Ca + + | Acetate 0.5 | 1.1813 | 0.12280 | 0.01287 | 1.1557 | 1.2069 | |||
| Citrate | 1.0871 | 0.02823 | 0.00685 | 1.0725 | 1.1016 | ||||
| Low acetate | 1.1617 | 0.10602 | 0.02164 | 1.1169 | 1,2064 | ||||
| Total | 1.1656 | 0.11579 | 0.01008 | 1.1457 | 1.1855 | 5,059 | 0.008 | ||
| P | Acetate 0.5 | 4.6548 | 1.48254 | 0.15373 | 4.3495 | 4.9602 | |||
| Citrate | 4.5412 | 0.96505 | 0.23406 | 4.0450 | 5.0374 | ||||
| Low acetate | 5.1308 | 1.61933 | 0.31758 | 4.4767 | 5.7848 | ||||
| Total | 4.7316 | 1.46053 | 0.12524 | 4.4839 | 4.9793 | 2.815 | 0.063 | ||
| PTH | Acetate 0.5 | 440.8200 | 343.63986 | 48.59802 | 343.1586 | 538.4814 | |||
| Citrate | 544.5625 | 223.08383 | 55.77096 | 425.6895 | 663.4355 | ||||
| Low acetate | 364.4500 | 203.08813 | 45.41189 | 269.4018 | 459.4982 | ||||
| Total | 442.3605 | 299.11870 | 32.25480 | 378.2293 | 506.4916 | 6.937 | 0.001 | ||
| Bic | Acetate 0.5 | 21.9854 | 3.36721 | 0.35692 | 21.2761 | 22.6947 | |||
| Citrate | 20.3563 | 2.54741 | 0.63685 | 18.9988 | 21.7137 | ||||
| Low acetate | 21.8292 | 3.28957 | 0.67148 | 20.4401 | 23.2182 | ||||
| Total | 21.7543 | 3.28365 | 0.28911 | 21.1822 | 22.3263 | 0.393 | 0.676 | ||
| Alb | Acetate 0.5 | 2.9441 | 0.61086 | 0.06334 | 2.8183 | 3.0699 | |||
| Citrate | 3.1529 | 0.53282 | 0.12923 | 2.8790 | 3.4269 | ||||
| Low acetate | 3.2269 | 0.53408 | 0.10474 | 3.0112 | 3.4426 | ||||
| Total | 3.0243 | 0.59597 | 0.05110 | 2.9232 | 3.1253 | 5.059 | 0.008 | ||
The 5 formulas are grouped into 3 types of LD: type 1 (acetate 0.5, Mg 0.5 mmol/l), type 3 (low acetate, Mg 0.37 mmol/l) and type 2 (citrate, Mg 0.5 mmol/l).
Albumin g/dl; Bicarbonate mmol/l; Ca + + mmol/l; Total Ca (Ca t) mg/dl; Mg mg/dl; P mg/dl; PTH pg/ml.
In bold, statistically significant, p < 0.05.
Patients on treatment with proton pump inhibitors (PPI), n = 102, (74%) show serum Mg no different from those who did not take it, n = 35, (26%).
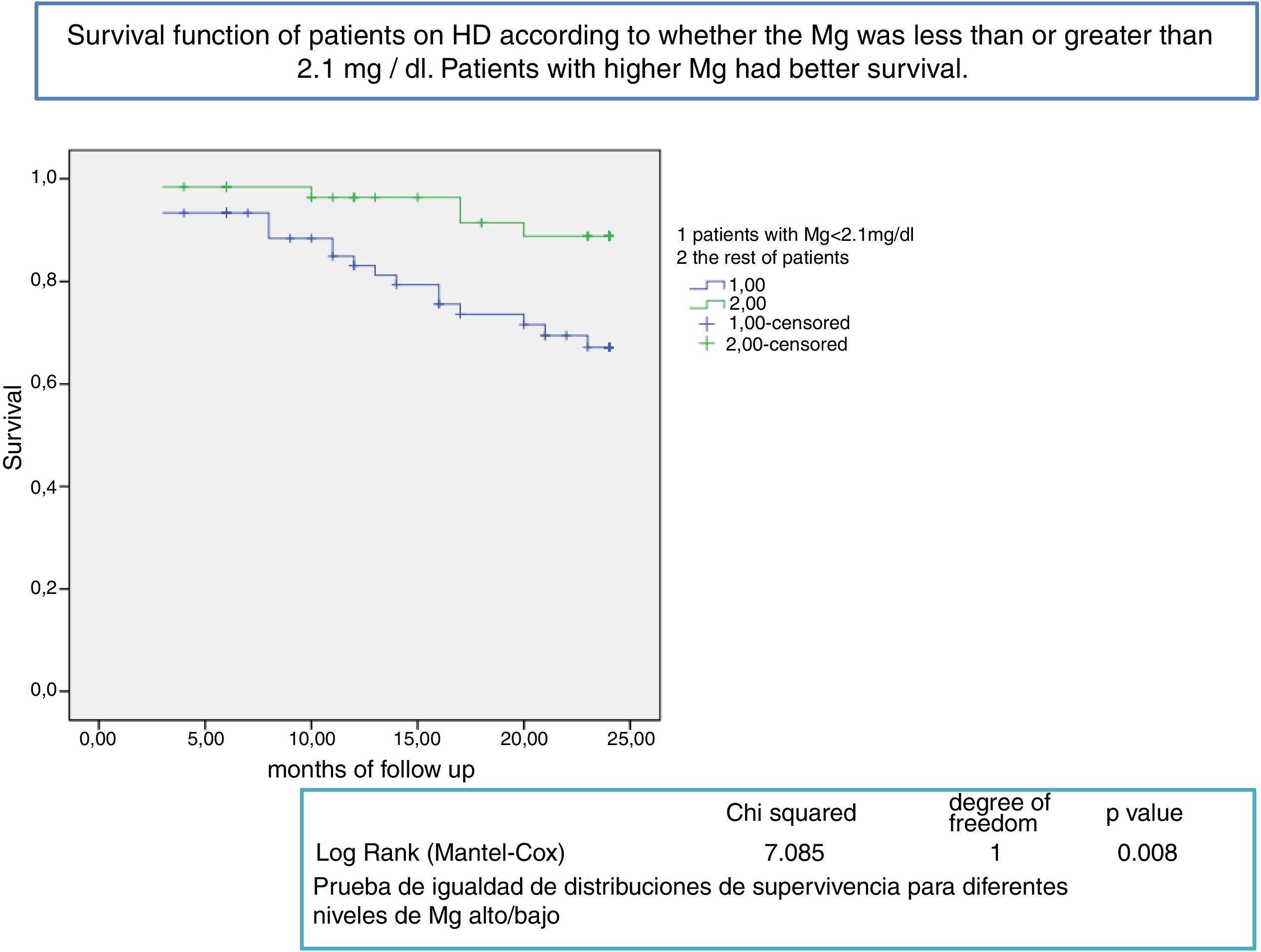
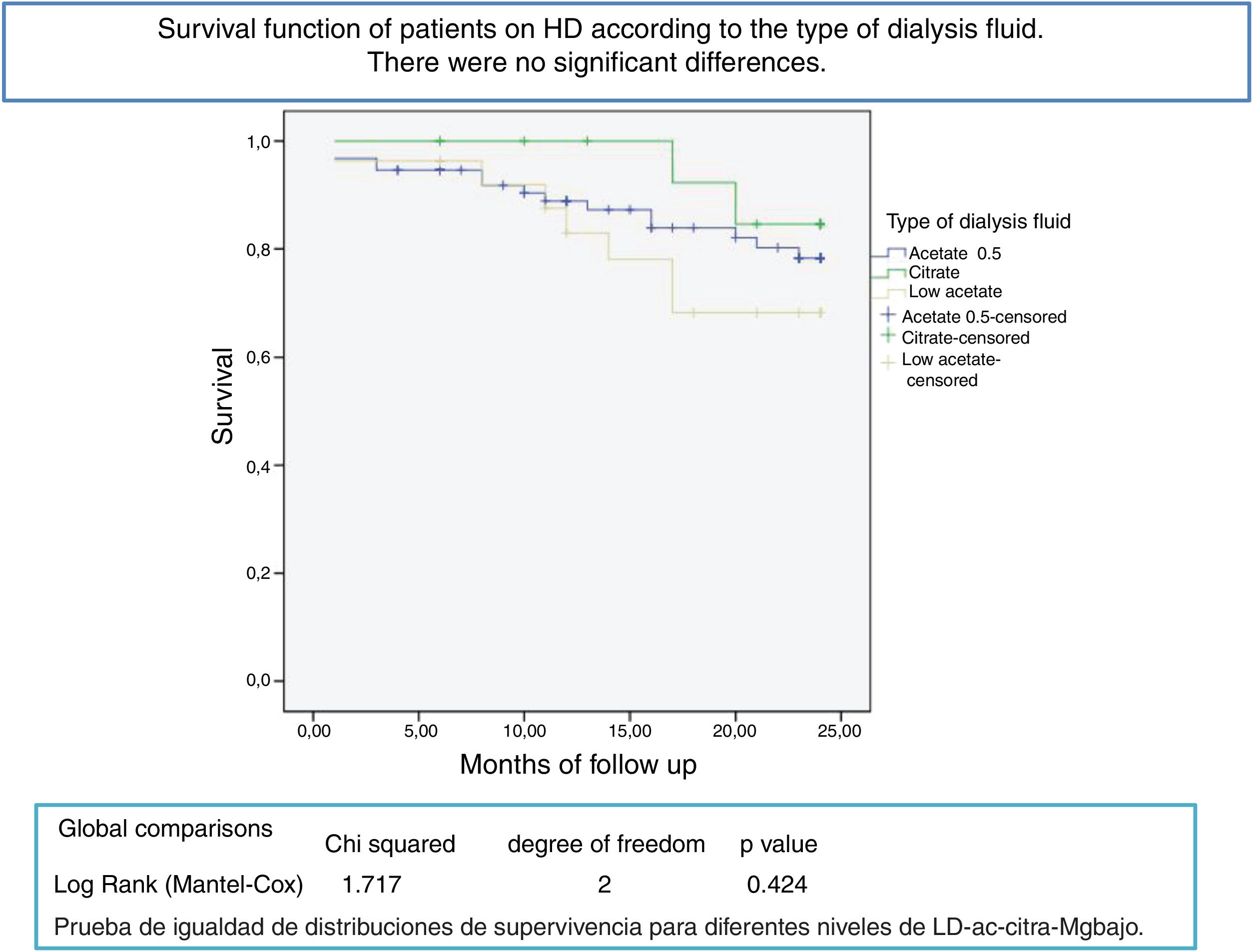
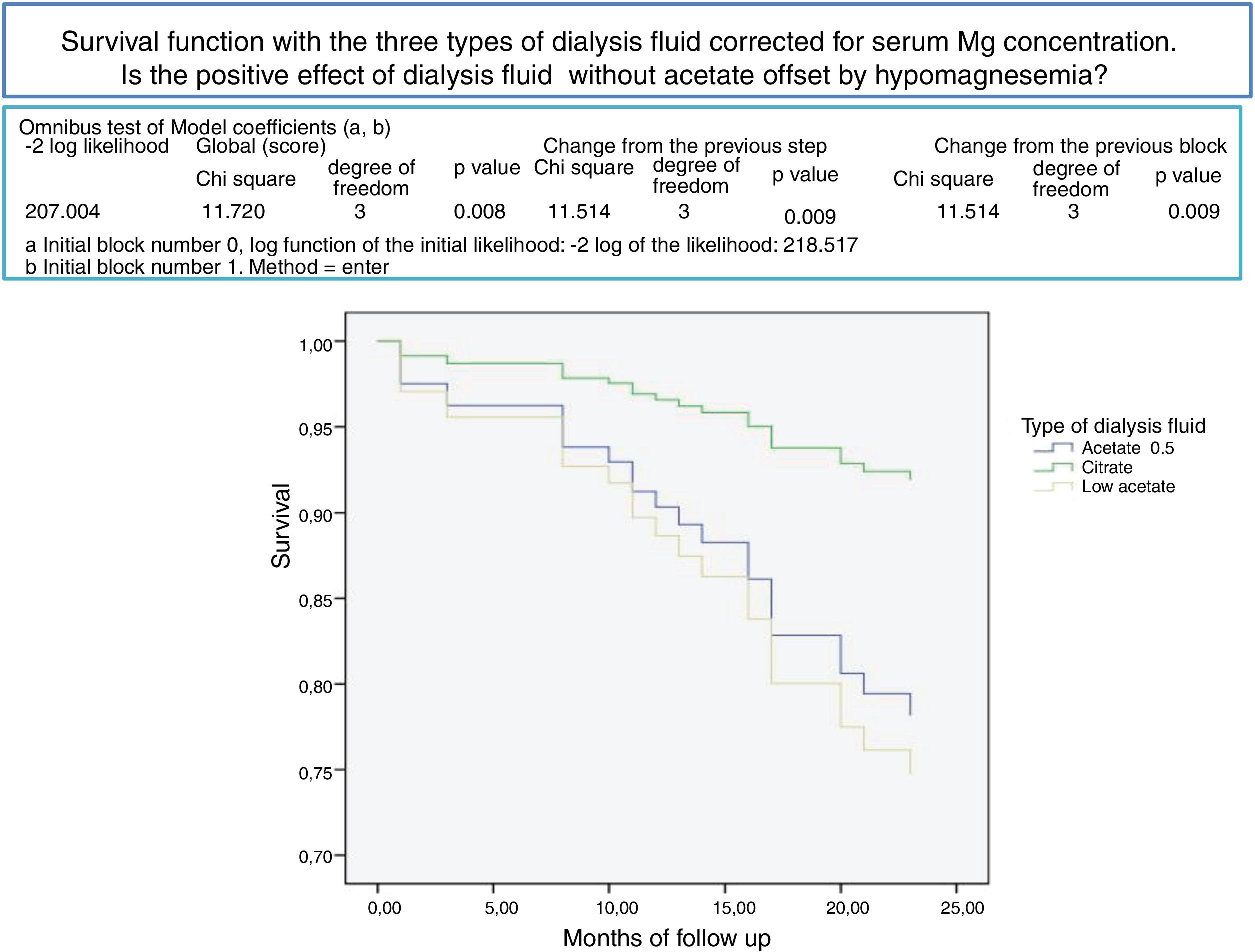
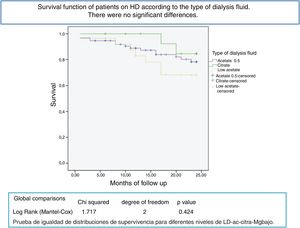
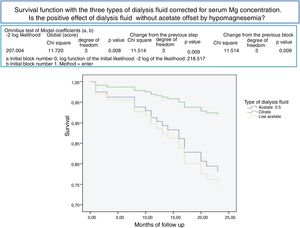
After a mean follow-up of 16 [3–24] months,8,9 77 patients remain still active, 24 had died and 36 had been transplanted or transferred. Patients with a Mg greater than 2.1 mg/dl have a longer survival than those with a lower Mg (Fig. 2). Survival of patients with the three types of dialysis fluid did not differ significantly, Log-Rank, p = 0.424 (Fig. 3). If the effect of the type of dialysis fluid on mortality is corrected the level of Mg, patients with citrate, dialysis fluid type 2, have better survival than those dialyzed with acetate (Fig. 4).
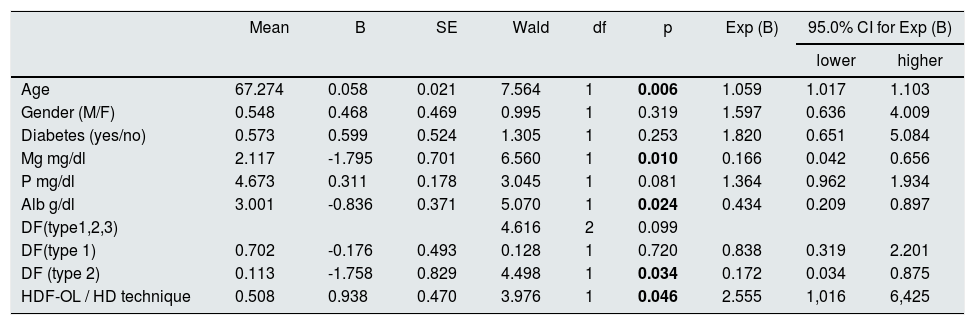
Table 4 shows that age, albumin, Mg, dialysis technique and type of dialysis fluid have an independent predictive effect on mortality.
COX survival analysis. Global score: gl 9, chi square 31.098; p = 0.00.
| Mean | B | SE | Wald | df | p | Exp (B) | 95.0% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|---|---|
| lower | higher | ||||||||
| Age | 67.274 | 0.058 | 0.021 | 7.564 | 1 | 0.006 | 1.059 | 1.017 | 1.103 |
| Gender (M/F) | 0.548 | 0.468 | 0.469 | 0.995 | 1 | 0.319 | 1.597 | 0.636 | 4.009 |
| Diabetes (yes/no) | 0.573 | 0.599 | 0.524 | 1.305 | 1 | 0.253 | 1.820 | 0.651 | 5.084 |
| Mg mg/dl | 2.117 | -1.795 | 0.701 | 6.560 | 1 | 0.010 | 0.166 | 0.042 | 0.656 |
| P mg/dl | 4.673 | 0.311 | 0.178 | 3.045 | 1 | 0.081 | 1.364 | 0.962 | 1.934 |
| Alb g/dl | 3.001 | -0.836 | 0.371 | 5.070 | 1 | 0.024 | 0.434 | 0.209 | 0.897 |
| DF(type1,2,3) | 4.616 | 2 | 0.099 | ||||||
| DF(type 1) | 0.702 | -0.176 | 0.493 | 0.128 | 1 | 0.720 | 0.838 | 0.319 | 2.201 |
| DF (type 2) | 0.113 | -1.758 | 0.829 | 4.498 | 1 | 0.034 | 0.172 | 0.034 | 0.875 |
| HDF-OL / HD technique | 0.508 | 0.938 | 0.470 | 3.976 | 1 | 0.046 | 2.555 | 1,016 | 6,425 |
DF: dialysis fluid. In bold, statistically significant, p < 0.05.
The present study shows that hemodialysis patients with serum Mg concentrations greater than mean of the population have a better survival than patients with values of Mg below the mean. Similar result has been observed in previous studies.16–22 In these studies, patients separated into two or three categories were compared according to the Mg concentrations. The patients with the best prognosis were those with high serum Mg concentration. In the work by Ishimura et al16 the cut-off point for Mg concentration favoring survival was 2.77 mg/dl of Mg. In another study in non-diabetic patients, the cut-off point was 2.5 mg/dl,20 which is higher than the value of 2.1 mg/dl obtained in our study. In the present study, the mean serum Mg concentration is lower than in others, probably due to the use in some patients of dialysis fluid with low Mg or with citrate. After the analysis of our results, our patients have not been dialyzed with a low Mg concentration in the dialysis fluid and higher Mg has been requested for some patients with acetate and for all patients on citrate.
The better survival of patients with high Mg in HD has been associated with a better nutritional status, with higher concentrations of albumin, triglycerides, phosphorus and lower values of CRP19 and with a lower degree of inflammation.18,22,31 Yu et al.22 observed that serum concentrations of albumin, urea, creatinine and uric acid were higher in patients with hypermagnesemia and the CRP and Lipoprotein A were lower.
In the work of Mizuiri et al31 the independent predictive value of Mg concentration on mortality is lost if albumin is included in the analysis which would mean for the authors that its role would be held in the nutritional status of the patient. In our study, both Mg and Albumin concentration maintains its independent predictive value for mortality, suggesting some other beneficial effect of Mg apart from the nutritional status, such as vascular calcifications and cardiac arrhythmias.
Why do some HD patients maintain a higher serum Mg than others?In some of the previous studies it was deduced that a better nutritional state is related to higher levels of Mg. The intake of Mg is associated with foods recommended in a healthy diet.11,12 The intake of Mg is currently decreasing and may not be sufficient to maintain the magnesium levels required to prevent diseases.11 The diet that is usually recommended for chronic kidney disease (CKD) patients, before or after initiation of regular HD is focused on K, P and proteins and not on Mg, and its content is often low. Adequate and sufficient intake will contribute to higher levels of Mg and better nutritional status.
In other studies, a higher concentration of serum Mg was due to treatment with MgO.19 Treatment with P binders containing Mg could contribute to higher levels of Mg.15,32 A reduced concentration of Mg is associated with treatment with PPI drugs.33–35 In our case, it was not observed the association of low Mg with PPI drugs, probably because it is masked by the lower concentrations of Mg induced by some of the dialysis fluids.
As observed in our study in HD patients, the concentration of Mg in the dialysis fluids and the presence of acetate or citrate would be determinants of the serum Mg concentration. Others have shown that increasing Mg concentration in the dialysis fluid results in an elevation of serum Mg concentration 36.
In dialysis patients, the ionic Mg / total Mg ratio is decreased. That is, “normal” magnesium values in the general population may be indicative of true hypomagnesemia in HD patients 37. This would be another argument to raise the content of magnesium in the Dialysis fluid. Alterations in the acid-base balance could influence the free fraction of magnesium.
Symptoms due to hypermagnesemia develop with concentration above 1.5 mmol/l (3.6 mg/dl); with values above 2.5 mmol/l there is areflexia and above 4.5 mmol/l respiratory or cardiac arrest may occur. These extremely high levels should be prevented, and generally caused by acute poisoning due to dialysate fluid contamination 38, or the massive intake of Epson's salt 39. For the treatment of eclampsia, the therapeutic limits would be between 2.06 and 3.7 mmol/l.40
Effects of Mg that may influence the prognosis of the patient in HDAs in the general population, the association of serum Mg with mortality has also been described in patients with (CKD) not on dialysis.41,42 Low serum Mg levels and low dietary magnesium intake would be associated with an increase in the incidence of CKD and its progression to advanced CKD.42 This could be due to the association of Mg with other factors that favor CKD progression and it may also be due to direct effects through endothelial dysfunction and vascular calcifications.
With respect to the HD treatment there are other factors that should be commented:
A relationship between the number of hypotensions and hypomagnesemia has been described.43 Hypotension on dialysis is a determinant of morbidity and mortality.
In animal models it has been demonstrated the role of Mg on inflammation.44 In HD patients there is an inverse relationship between CRP and Mg suggesting a role of low Mg on inflammation.18 Thus, hypomagnesemia would be another of the factors involved in the chronic inflammatory status of HD patients.
In uremic rats, increasing Mg intake reduces mortality and reduce vascular calcifications.45 The initiation of calcification by the complex Ca-PL-PO4 is dependent on the Mg / Ca ratio present in the calcifying tissue.46 Mg, by preventing the maturation of the calciprotein particles, would inhibit the calcification of the smooth muscle cells of the vessels induced by high P.42 The risk of increased mortality associated with hyperphosphatemia would be attenuated by high Mg concentrations.47 Serum Mg would be an independent predictor of the non-progression of vascular calcifications.48 Increasing Mg in the dialysis fluid from 1.0 to 2.0 mEq / L decreases the rate of calcification in HD patients.49
Mg is capable of modulating PTH but with a lower potency than Ca. Hypermagnesemia could be associated with lower levels of PTH.50 Mild hypermagnesemia would be associated with a lower risk of fractures in HD patients.51 Mg has always been the great forgotten player of bone and mineral metabolism alterations.52
Changes in Mg along with modifications of K and Ca are associated with QT prolongation and the occurrence of cardiac arrhythmias.53,54 Severe cardiac arrhythmias are a major cause of mortality in HD.
High concentrations of serum Mg would be related to a better response to erythropoietin (EPO).55 The response index to erythropoietin is a prognostic marker.
Muscle function would be altered in HD patients with hypomagnesemia.56
All these effects of a low concentration of Mg could explain the relationship between Mg and mortality in HD patients.
Dialysis fluid (LD) and its influence on Mg and mortality in HDThe concentrations of Acetate in dialysis fluid commonly used in clinical practice, 3-4 mmol / l, increases the oxidative stress, the production of pro-inflammatory cytokines and synthesis of nitric oxide and may act as an adjunct to the other pro-inflammatory stimuli of uremic patients on HD.57 The elimination of acetate from HD could improve survival of HD patients, at least in those older than 70 years.58 Dialysis fluid with Citrate (CDF) do not produce this activation, so it could be an alternative in clinical practice. Citrate dialysis produces less acute post-dialysis alkalemia and modifies significantly serum levels of Ca, Mg and PTH.30,59 CDF has a positive impact on hemodynamic tolerance.30 Citrate in the dialysis fluid produces a negative balance of Ca and Mg26–29 so it could induce hypomagnesemia in some patients.39 The beneficial effect of a change of acetate by citrate could be counteracted by the development of hypomagnesemia. In the present work, the three types of dialysis fluids do not significantly influence mortality, but when the citrate dialysis fluid is associated with other variables and corrected for magnesium, it is associated with better survival. In the CDF, CX 265 G (Table 1) the concentration of Ca has been increased to 1.65 mmol/l to counteract the lower balance of Ca with citrate, the same should be done with Mg, which should at least be increased to 0.75 mmol/l. This would also be valid for some patients on dialysis fluid containing acetate; Schmaderer et al.36 showed that by increasing the Mg concentration in the dialysis fluid to 0.75 mmol/l there is an improvement of cardiovascular mortality. The routine use of 0.5 mmol/l of Mg in dialysate with acetate would cause a slow decrease in serum Mg in most patients.60
Limitation of the studyBeing a single center study the total number of patients recruited resulted to be low. This small number of patients prevented the inclusion of more variables in the regression model, although all patients being treated with the same guidelines reduces of this limitation.
ConclusionsPredialysis serum Mg concentrations above 2.1 mg/dl are associated with lower mortality than those with lower concentrations.
Dialysis fluids with Mg 0.37 mmol/l and acetate and with Mg 0.5 mmol/l and citrate are associated with low magnesium levels.
Dialysis fluids with citrate and Mg 0.5 mmol/l reduces the serum Mg concentration in the patient which neutralizes the possible beneficial effect of the dialysis fluid with citrate.
Hypothesis — future studiesIt is postulated that the Mg content in the LD should be >0.5 mmol/l in some patients.
In the dialysis fluid with citrate, the Mg could be 0.75 mmol/l.Hypothesis 1 An increase in the concentration of Mg above 0.5 mmol/l in the dialysis fluid with citrate could improve the clinical results. some patients with low Mg could benefit from a concentration greater than 0.5 mmol/L of Mg in the acetate dialysis fluid.
The authors declare that they have no conflict of interest.
Please cite this article as: Pérez-García R, Jaldo MT, Puerta M, Ortega M, Corchete E, de Sequera P, et al. La hipomagnesemia en hemodiálisis se asocia a mayor riesgo de mortalidad: su relación con el líquido de diálisis. Nefrologia. 2020;40:552–562.