The most common adverse effect of baclofen, used for managing hiccups and spasticity, is neurotoxicity. As baclofen is primarily excreted by the kidneys, neurotoxicity is more likely to occur in patients with chronic kidney disease (CKD). We evaluated the risk factor for baclofen neurotoxicity and the recommended dosage for patients with severe CKD.
MethodsIn this single-center retrospective study, we classified 401 patients with CKD as stage 4 (n=174), non-dialysis stage 5 (n=97), and on-dialysis (n=130).
ResultsThe prevalence of baclofen-induced neurotoxicity in patients with severe CKD was 7.0% (28 of 401 patients). There was no significant difference in the presence of neurotoxicity when the patients were classified as CKD stage 4, stage 5, and dialysis patients. There were significant differences in serum albumin levels and the presence of diabetic nephropathy between the patients with neurotoxicity and those without. The results from a multiple logistic regression analysis showed that serum albumin was independently associated with baclofen neurotoxicity (p=0.007). The minimum daily dose for baclofen neurotoxicity was 10mg, 10mg, and 5mg in patients with CKD stages 4 and 5, and dialysis, respectively.
ConclusionsIn this study, the prevalence of baclofen-induced neurotoxicity in patients with severe CKD was 7.0%. Serum albumin was identified as an independent risk factor for neurotoxicity. We recommend initially administering a daily dose of 7.5mg for patients with severe CKD stages 4 and 5, and a daily dose of 2.5mg for patients receiving dialysis.
La neurotoxicidad es el efecto adverso más frecuente del baclofeno, un fármaco que se utiliza para tratar los espasmos y la espasticidad. Dado que el baclofeno se excreta principalmente a través de los riñones, es más probable que la neurotoxicidad se presente en pacientes con enfermedad renal crónica (ERC). Hemos evaluado el factor de riesgo para la neurotoxicidad por baclofeno y la dosis recomendada para pacientes con ERC grave.
MétodosEn este estudio retrospectivo unicéntrico, se clasificó a 401 pacientes con ERC en estadio 4 (n=174), estadio 5 sin diálisis (n=97) y en diálisis (n=130).
ResultadosLa prevalencia de la neurotoxicidad inducida por baclofeno en pacientes con ERC grave fue del 7,0% (28 de 401 pacientes). No se observaron diferencias significativas en la presencia de neurotoxicidad al clasificar a los pacientes en ERC en estadio 4, estadio 5 y pacientes en diálisis. Se observaron diferencias significativas en los niveles de albúmina sérica y en la presencia de nefropatía diabética entre los pacientes con y sin neurotoxicidad. Los resultados de un análisis de regresión logística múltiple mostraron que la albúmina sérica estaba asociada de manera independiente a la neurotoxicidad por baclofeno (p=0,007). La dosis diaria mínima para la neurotoxicidad por baclofeno fue de 10, 10 y 5mg en pacientes con ERC en estadio 4, estadio 5 y en diálisis, respectivamente.
ConclusionesEn este estudio, la prevalencia de la neurotoxicidad inducida por baclofeno en pacientes con ERC grave fue del 7,0%. Se identificó la albúmina sérica como un factor de riesgo independiente de neurotoxicidad. Recomendamos una administración inicial a una dosis diaria de 7,5mg en pacientes con ERC grave en estadios 4 y 5, y una dosis diaria de 2,5mg en pacientes que reciben diálisis.
Baclofen is a derivative of γ-aminobutyric acid (GABA), which acts mainly on the vertebral GABA-B receptors to inhibit synaptic motor neurons, thereby resulting in a central anticonvulsant response.1,2 The GABA-B receptor, which is associated with the G protein, opens the nearby potassium channel, hence increasing the potassium membrane conductivity, promoting cell membrane polarization, and preventing action potentials from occurring.3 Baclofen is used for treating spastic movement disorders, such as spinal cord injury, cerebral palsy, and multiple sclerosis. It is also used for managing uncontrolled hiccups (>48h) in patients with cancer or patients undergoing alcohol and drug withdrawal symptoms.1,3–11
The therapeutic dose of baclofen is 15–80mg/day, and the plasma concentration is 80–400ng/mL.6,12,13 The half-life of baclofen is 2–6h, the molecular weight is 213Da, and the volume of distribution is 0.8L/kg in adults and 2.6L/kg in children.1,3,7,12,14–16 Also, it is moderately lipophilic and approximately 30% binds to serum protein.12 It is well distributed in the vascular space and blood-rich organs (liver and kidney), but it is distributed at a low plasma concentration of 12% in the central nervous system (CNS), as it passes through the blood–brain barrier.4,5,12,13 Orally administered baclofen is rapidly absorbed in the digestive tract. Liver metabolism plays a small role. Approximately, 85% of this agent is excreted in the kidneys; so, if the patient's renal function is poor, the half-life of the agent will be long.1,3,6,7,14,15,17
Baclofen slowly passes through the blood-brain barrier and exhibits neurotoxicity.5,12 In the CNS, the removal of baclofen is also slow. Therefore, the CNS symptoms may persist even though the plasma baclofen concentration is decreased.3,13,15,18 The most common symptom is alteration in consciousness. Abdominal pain often occurs due to a cholinergic reaction mediated by GABA.4,18 At low doses of baclofen, patients might experience such symptoms as temporary drowsiness and nausea. A high-dose baclofen administration can cause CNS depression, such as sedation, drowsiness, and respiratory depression. Patients with a history of seizures may have more pronounced seizures.3 If baclofen-induced acute encephalopathy occurs, an electroencephalogram is requested, in which case, the results may reveal various patterns, such as periodic 3 phase complex; however, no special treatment is required.19–22
There is no specific antidote to baclofen toxicity, and hemodialysis is most commonly used for treating neurotoxicity because of its low-molecular-weight, lipophilic nature, and low binding to proteins.1,4,7,15,23 Dialysis can remove the drug from the body at the same rate as in people with normal renal function, and when dialyzed for 4h, about 79% can be eliminated, and the half-life can be reduced from 15h to 2h.15,24 Even in patients with normal renal function, baclofen overuse may cause neurotoxicity, and dialysis is also useful in this case.23
Baclofen is, for the most part, excreted in the kidneys; so, patients with acute kidney injury or chronic kidney disease (CKD) who do not reduce the dose will have symptoms, such as encephalopathy, respiratory failure, and unconsciousness.3,6 In many cases, the symptom appears as iatrogenic, and there is room for prevention. However, there is still no standard for drug dosage reduction according to renal function, which makes it difficult to control the drug.3,5,25 Previous studies have shown that even a small amount of baclofen (5mg) may cause neurotoxicity, but the greater the dose, the older the age, and the more extensive the brain lesion, the greater the chance of neurotoxicity.1,7,12,18,26 It is also reported that neurotoxicity is more pronounced in men (56.3%), patients with end-stage renal disease who receive dialysis (62.9%), or people with concomitant use of CNS depressants.1,16,27 There were no data on the prevalence of neurotoxicity.
In this study, we analyzed the prevalence and the risk factors of neurotoxicity in patients with severe CKD who were treated with baclofen at XXXX Medical Center for the last 11 years.
MethodsPatientsWe retrospectively studied CKD stage 4, non-dialysis stage 5, and on-dialysis patients, who were treated with baclofen at XXXX Medical Center from January 1, 2006, to December 31, 2016. A search using the XXXX Biomedical Research Environment revealed that 17,041 patients were treated with baclofen during this period. Of the total patients, 523 patients had a Modification of Diet in Renal Disease estimated Glomerular Filtration Rate (MDRD-eGFR) of <30mL/min/1.73m2 and were assigned the disease code N18* ICD-9-CM (except N181, N182, and N183, which correspond to CKD stages 1, 2, and 3, respectively). We extracted the data on these patients with MDRD-eGFR of <30mL/min/1.73m2 measured at the closest date to the last baclofen prescription. Consequently, we extracted the data on 401 of 523 individuals, and we analyzed and compared their information. This study is a retrospective analysis of the medical records. Because there is little likelihood of any type of infringement on the patients’ human rights, we did not obtain the patients’ informed consent.
Data collectionThe following data were collected:
- 1.
Baseline characteristics of patients: sex, age, height, weight, and body mass index (BMI)
- 2.
Underlying diseases: diabetic nephropathy, hypertension, congestive heart failure, cancer, and CNS disease
- 3.
Baclofen medication information
- (1)
Reason for medication: hiccups, muscle cramps, others
- (2)
Prescribed place: inpatient, outpatient
- (3)
Total dose
- (4)
Medication period
- (5)
Daily dose (total dose/medication period)
- 4.
Clinical features of patients with neurotoxicity
- (1)
Neurotoxic symptoms
- (1)
Mental change
- (2)
Seizure
- (3)
Time to symptom onset
- (4)
Duration of symptoms
- (2)
Neurotoxicity treatment
- (1)
- (1)
Intensive care unit (ICU): Yes/No
- (2)
ICU duration
- (3)
Use of ventilator: Yes/No
- (4)
Ventilator duration
- (5)
Therapeutic hemodialysis, number of times
- (6)
Therapeutic peritoneal dialysis, days
- 5.
Taking other drugs acting on the CNS: Yes/No
- 6.
Laboratory results
- (1)
Serum creatinine
- (2)
Serum MDRD-eGFR
- (3)
Serum albumin
- (4)
Serum cholesterol
- (5)
Serum C-reactive protein (CRP)
- (6)
Serum total CO2
- (7)
Plasma pCO2
- (8)
Plasma bicarbonate
Baclofen neurotoxicity was defined as a state in which neurological symptoms, such as mental alterations, seizures, encephalopathy, and respiratory depression, which occurred after baclofen administration could not be explained by causes other than baclofen. The laboratory results at the time of neurotoxicity diagnosis were documented for patients with neurotoxicity, and for patients without neurotoxicity, the laboratory results from the last day of baclofen administration were recorded. When baclofen was prescribed several times during the study period, the total dose was defined as the total dose at the time of the first prescription period. Severe CKD was defined as a state in which the renal function was stage 4 or lower.
Ethics statementsThe study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in Brazil, 2013). The protocol was approved by an Institutional Review Board of XXXXX XXXX Medical Center (No. 2017-0429).
Statistical analysisThe numerical data were expressed as the mean±standard deviation values when the normality was satisfied, and as the median, minimum, and maximum values when the normality was not satisfied. The frequency of occurrence was expressed as a percentage (%). The normality test was performed using the Kolmogorov–Smirnov test. Student's t-test was used for comparison between two independent groups that met the normality. The difference between the three groups was analyzed using one-way analysis of variance. The Mann–Whitney U test was used for comparison between two independent groups that did not satisfy the normality, and the Kruskal–Wallis test was used for comparison between the three groups. The categorical data were analyzed using the chi-squared test. A multiple logistic regression analysis was used to evaluate the independent association of neurotoxicity with significant factors. All of the analyses were performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA), and p<0.05 was considered to be statistically significant.
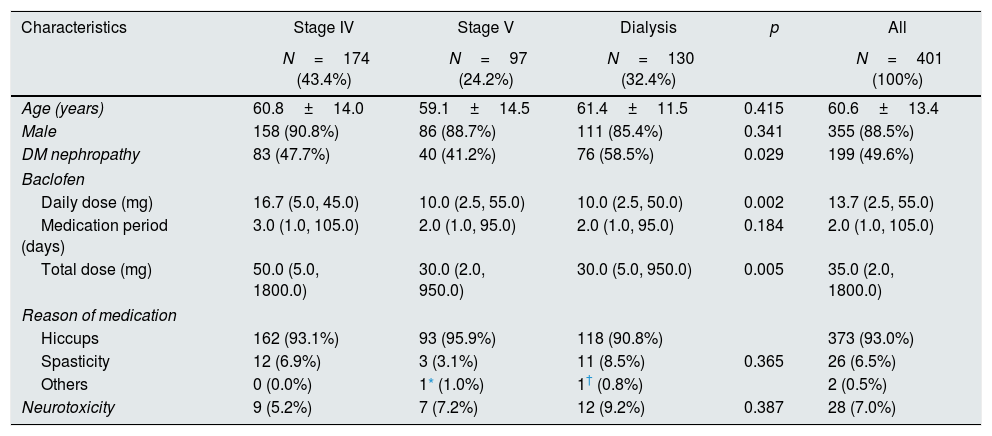
ResultsComparison of neurotoxicity with the severity of CKDOf the 401 patients, 355 (88.5%) patients were male and 46 (11.5%) patients were female. CKD stage 4, non-dialysis stage 5, and on-dialysis patients did not differ in age, sex, medication period, reason for medication, and neurotoxicity. There was a difference in diabetic nephropathy (p=0.029), daily dose (p=0.002), and total dose (p=0.005) (Table 1).
Patients’ characteristics and prevalence of neurotoxicity according to the CKD stages.
| Characteristics | Stage IV | Stage V | Dialysis | p | All |
|---|---|---|---|---|---|
| N=174 (43.4%) | N=97 (24.2%) | N=130 (32.4%) | N=401 (100%) | ||
| Age (years) | 60.8±14.0 | 59.1±14.5 | 61.4±11.5 | 0.415 | 60.6±13.4 |
| Male | 158 (90.8%) | 86 (88.7%) | 111 (85.4%) | 0.341 | 355 (88.5%) |
| DM nephropathy | 83 (47.7%) | 40 (41.2%) | 76 (58.5%) | 0.029 | 199 (49.6%) |
| Baclofen | |||||
| Daily dose (mg) | 16.7 (5.0, 45.0) | 10.0 (2.5, 55.0) | 10.0 (2.5, 50.0) | 0.002 | 13.7 (2.5, 55.0) |
| Medication period (days) | 3.0 (1.0, 105.0) | 2.0 (1.0, 95.0) | 2.0 (1.0, 95.0) | 0.184 | 2.0 (1.0, 105.0) |
| Total dose (mg) | 50.0 (5.0, 1800.0) | 30.0 (2.0, 950.0) | 30.0 (5.0, 950.0) | 0.005 | 35.0 (2.0, 1800.0) |
| Reason of medication | |||||
| Hiccups | 162 (93.1%) | 93 (95.9%) | 118 (90.8%) | 373 (93.0%) | |
| Spasticity | 12 (6.9%) | 3 (3.1%) | 11 (8.5%) | 0.365 | 26 (6.5%) |
| Others | 0 (0.0%) | 1* (1.0%) | 1† (0.8%) | 2 (0.5%) | |
| Neurotoxicity | 9 (5.2%) | 7 (7.2%) | 12 (9.2%) | 0.387 | 28 (7.0%) |
CKD, chronic kidney disease; DM, diabetes mellitus.
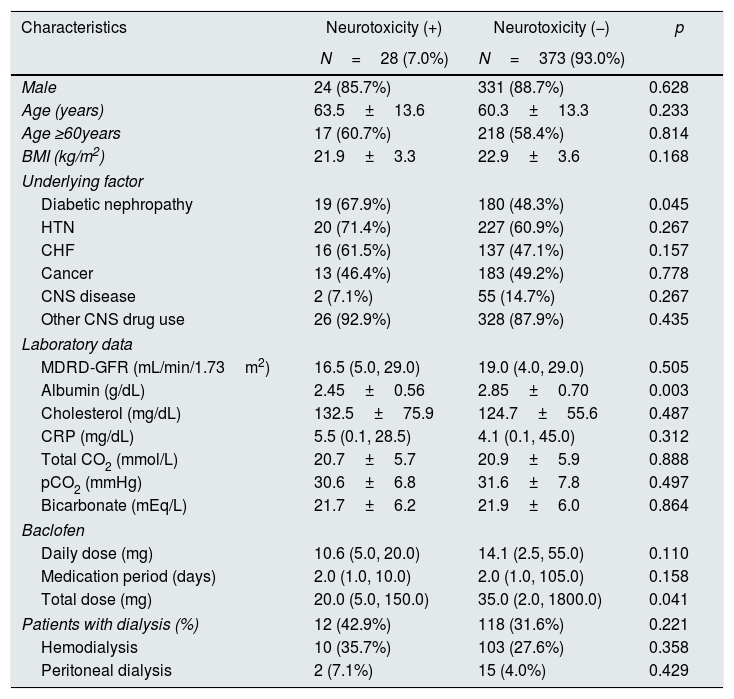
We investigated whether the incidence of neurotoxicity was related to the patient's baseline characteristics, underlying disease, laboratory data, baclofen dose and duration, and dialysis. Diabetic nephropathy (p=0.045), serum albumin (p=0.003), and baclofen total dose (p=0.041) were associated with neurotoxicity. Other factors were not associated with neurotoxicity (Table 2).
Patients’ characteristics according to the presence of neurotoxicity.
| Characteristics | Neurotoxicity (+) | Neurotoxicity (−) | p |
|---|---|---|---|
| N=28 (7.0%) | N=373 (93.0%) | ||
| Male | 24 (85.7%) | 331 (88.7%) | 0.628 |
| Age (years) | 63.5±13.6 | 60.3±13.3 | 0.233 |
| Age ≥60years | 17 (60.7%) | 218 (58.4%) | 0.814 |
| BMI (kg/m2) | 21.9±3.3 | 22.9±3.6 | 0.168 |
| Underlying factor | |||
| Diabetic nephropathy | 19 (67.9%) | 180 (48.3%) | 0.045 |
| HTN | 20 (71.4%) | 227 (60.9%) | 0.267 |
| CHF | 16 (61.5%) | 137 (47.1%) | 0.157 |
| Cancer | 13 (46.4%) | 183 (49.2%) | 0.778 |
| CNS disease | 2 (7.1%) | 55 (14.7%) | 0.267 |
| Other CNS drug use | 26 (92.9%) | 328 (87.9%) | 0.435 |
| Laboratory data | |||
| MDRD-GFR (mL/min/1.73m2) | 16.5 (5.0, 29.0) | 19.0 (4.0, 29.0) | 0.505 |
| Albumin (g/dL) | 2.45±0.56 | 2.85±0.70 | 0.003 |
| Cholesterol (mg/dL) | 132.5±75.9 | 124.7±55.6 | 0.487 |
| CRP (mg/dL) | 5.5 (0.1, 28.5) | 4.1 (0.1, 45.0) | 0.312 |
| Total CO2 (mmol/L) | 20.7±5.7 | 20.9±5.9 | 0.888 |
| pCO2 (mmHg) | 30.6±6.8 | 31.6±7.8 | 0.497 |
| Bicarbonate (mEq/L) | 21.7±6.2 | 21.9±6.0 | 0.864 |
| Baclofen | |||
| Daily dose (mg) | 10.6 (5.0, 20.0) | 14.1 (2.5, 55.0) | 0.110 |
| Medication period (days) | 2.0 (1.0, 10.0) | 2.0 (1.0, 105.0) | 0.158 |
| Total dose (mg) | 20.0 (5.0, 150.0) | 35.0 (2.0, 1800.0) | 0.041 |
| Patients with dialysis (%) | 12 (42.9%) | 118 (31.6%) | 0.221 |
| Hemodialysis | 10 (35.7%) | 103 (27.6%) | 0.358 |
| Peritoneal dialysis | 2 (7.1%) | 15 (4.0%) | 0.429 |
BMI, body mass index; HTN, hypertension; CHF, congestive heart failure; CNS, central nervous system; MDRD-GFR, Modification of Diet in Renal Disease estimated glomerular filtration rate; CRP, C-reactive protein; pCO2, partial pressure of carbon dioxide.
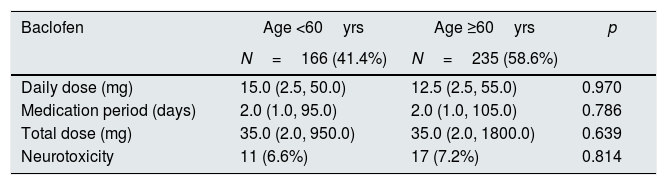
The patients were divided into two groups: those younger than 60 years of age and those older than 60 years. Baclofen doses did not differ between the daily doses, the dosing duration, and the total dose. There was no significant difference in the incidence of neurotoxicity (Table 3).
Baclofen dose and prevalence of neurotoxicity according to patient's age.
| Baclofen | Age <60yrs | Age ≥60yrs | p |
|---|---|---|---|
| N=166 (41.4%) | N=235 (58.6%) | ||
| Daily dose (mg) | 15.0 (2.5, 50.0) | 12.5 (2.5, 55.0) | 0.970 |
| Medication period (days) | 2.0 (1.0, 95.0) | 2.0 (1.0, 105.0) | 0.786 |
| Total dose (mg) | 35.0 (2.0, 950.0) | 35.0 (2.0, 1800.0) | 0.639 |
| Neurotoxicity | 11 (6.6%) | 17 (7.2%) | 0.814 |
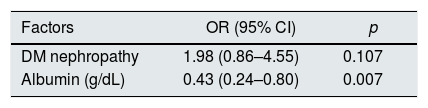
Diabetic nephropathy and serum albumin, which were associated with neurotoxicity, were analyzed using a multiple logistic regression analysis. As a result, serum albumin was independently associated with baclofen neurotoxicity (p=0.007), and diabetic nephropathy was not independently associated (Table 4).
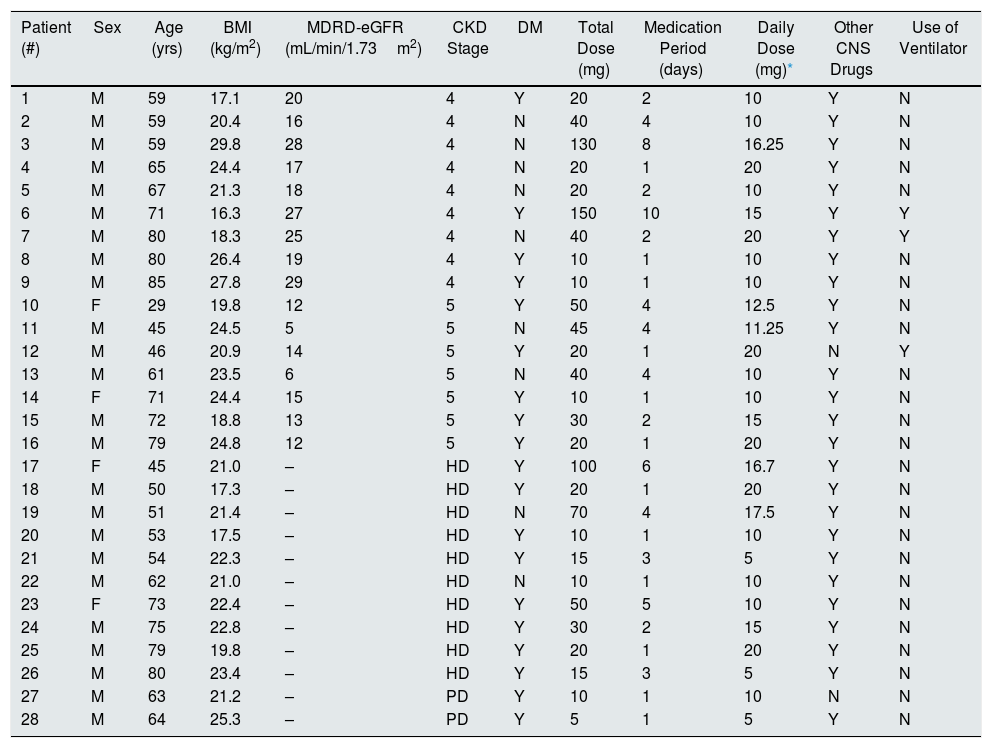
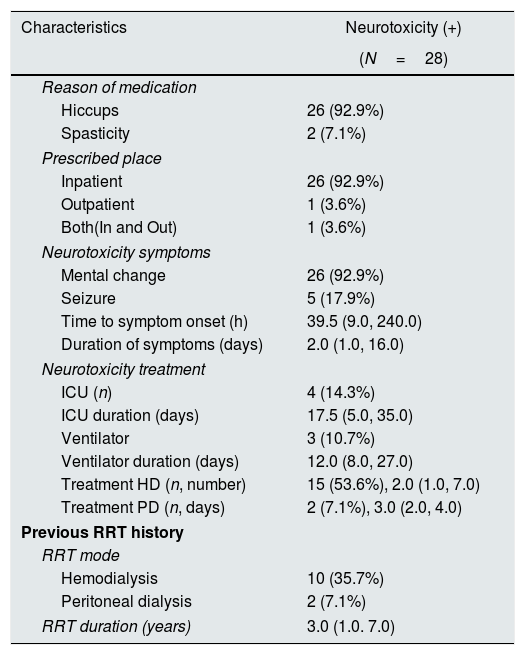
Clinical characteristics of patients with neurotoxicityTwenty-six (92.9%) patients received baclofen to treat hiccups, and two (7.1%) patients were treated for muscle spasms. Regarding the prescribed place, the inpatient prescription was the most common (92.9%). Mental alteration was present in 26 (92.9%) patients, and seizure occurred in five (17.9%) patients. The time to symptom onset was 39.5 (9.0, 240.0) h, and the duration of symptom was 2.0 (1.0, 16.0) days. Four (14.3%) patients received treatment in the ICU for 17.5 (5.0, 35.0) days. One patient (Table 5, #7) had community-acquired pneumonia and received ventilatory treatment in the ICU. He started taking baclofen to control hiccups. After 41h, complete sedation and loss of spontaneous breathing were observed, and there was no change in the sedation drug dose. There was no specific finding on brain imaging, and he recovered after 12 days of ceasing baclofen treatment. Three (10.7%) patients had used the ventilator for baclofen neurotoxicity for 12.0 (8.0, 27.0) days. A total of 15 (53.6%) patients underwent hemodialysis (number of dialysis, 2.0 [1.0, 7.0] times) for the treatment of baclofen neurotoxicity. One patient applied continuous renal replacement therapy in the ICU (Table 5, #12). Two (7.1%) patients underwent therapeutic peritoneal dialysis for 3.0 (2.0, 4.0) days (Table 27, #28). Patients who had been on dialysis continued to receive dialysis for 3.0 (1.0–7.0) years. Among them, 10 patients (35.7%) had hemodialysis, and two patients (7.1%) had peritoneal dialysis (Table 6).
Clinical characteristics of each patient with neurotoxicity.
| Patient (#) | Sex | Age (yrs) | BMI (kg/m2) | MDRD-eGFR (mL/min/1.73m2) | CKD Stage | DM | Total Dose (mg) | Medication Period (days) | Daily Dose (mg)* | Other CNS Drugs | Use of Ventilator |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 59 | 17.1 | 20 | 4 | Y | 20 | 2 | 10 | Y | N |
| 2 | M | 59 | 20.4 | 16 | 4 | N | 40 | 4 | 10 | Y | N |
| 3 | M | 59 | 29.8 | 28 | 4 | N | 130 | 8 | 16.25 | Y | N |
| 4 | M | 65 | 24.4 | 17 | 4 | N | 20 | 1 | 20 | Y | N |
| 5 | M | 67 | 21.3 | 18 | 4 | N | 20 | 2 | 10 | Y | N |
| 6 | M | 71 | 16.3 | 27 | 4 | Y | 150 | 10 | 15 | Y | Y |
| 7 | M | 80 | 18.3 | 25 | 4 | N | 40 | 2 | 20 | Y | Y |
| 8 | M | 80 | 26.4 | 19 | 4 | Y | 10 | 1 | 10 | Y | N |
| 9 | M | 85 | 27.8 | 29 | 4 | Y | 10 | 1 | 10 | Y | N |
| 10 | F | 29 | 19.8 | 12 | 5 | Y | 50 | 4 | 12.5 | Y | N |
| 11 | M | 45 | 24.5 | 5 | 5 | N | 45 | 4 | 11.25 | Y | N |
| 12 | M | 46 | 20.9 | 14 | 5 | Y | 20 | 1 | 20 | N | Y |
| 13 | M | 61 | 23.5 | 6 | 5 | N | 40 | 4 | 10 | Y | N |
| 14 | F | 71 | 24.4 | 15 | 5 | Y | 10 | 1 | 10 | Y | N |
| 15 | M | 72 | 18.8 | 13 | 5 | Y | 30 | 2 | 15 | Y | N |
| 16 | M | 79 | 24.8 | 12 | 5 | Y | 20 | 1 | 20 | Y | N |
| 17 | F | 45 | 21.0 | – | HD | Y | 100 | 6 | 16.7 | Y | N |
| 18 | M | 50 | 17.3 | – | HD | Y | 20 | 1 | 20 | Y | N |
| 19 | M | 51 | 21.4 | – | HD | N | 70 | 4 | 17.5 | Y | N |
| 20 | M | 53 | 17.5 | – | HD | Y | 10 | 1 | 10 | Y | N |
| 21 | M | 54 | 22.3 | – | HD | Y | 15 | 3 | 5 | Y | N |
| 22 | M | 62 | 21.0 | – | HD | N | 10 | 1 | 10 | Y | N |
| 23 | F | 73 | 22.4 | – | HD | Y | 50 | 5 | 10 | Y | N |
| 24 | M | 75 | 22.8 | – | HD | Y | 30 | 2 | 15 | Y | N |
| 25 | M | 79 | 19.8 | – | HD | Y | 20 | 1 | 20 | Y | N |
| 26 | M | 80 | 23.4 | – | HD | Y | 15 | 3 | 5 | Y | N |
| 27 | M | 63 | 21.2 | – | PD | Y | 10 | 1 | 10 | N | N |
| 28 | M | 64 | 25.3 | – | PD | Y | 5 | 1 | 5 | Y | N |
#, Patient number; BMI, body mass index; MDRD-eGFR, Modification of Diet in Renal Disease estimated glomerular filtration rate; CKD, chronic kidney disease; DM, diabetes mellitus; CNS, central nervous system; M, male; F, female; HD, hemodialysis; PD, peritoneal dialysis; Y, yes; N, no.
Clinical characteristics of patients with neurotoxicity.
| Characteristics | Neurotoxicity (+) |
|---|---|
| (N=28) | |
| Reason of medication | |
| Hiccups | 26 (92.9%) |
| Spasticity | 2 (7.1%) |
| Prescribed place | |
| Inpatient | 26 (92.9%) |
| Outpatient | 1 (3.6%) |
| Both(In and Out) | 1 (3.6%) |
| Neurotoxicity symptoms | |
| Mental change | 26 (92.9%) |
| Seizure | 5 (17.9%) |
| Time to symptom onset (h) | 39.5 (9.0, 240.0) |
| Duration of symptoms (days) | 2.0 (1.0, 16.0) |
| Neurotoxicity treatment | |
| ICU (n) | 4 (14.3%) |
| ICU duration (days) | 17.5 (5.0, 35.0) |
| Ventilator | 3 (10.7%) |
| Ventilator duration (days) | 12.0 (8.0, 27.0) |
| Treatment HD (n, number) | 15 (53.6%), 2.0 (1.0, 7.0) |
| Treatment PD (n, days) | 2 (7.1%), 3.0 (2.0, 4.0) |
| Previous RRT history | |
| RRT mode | |
| Hemodialysis | 10 (35.7%) |
| Peritoneal dialysis | 2 (7.1%) |
| RRT duration (years) | 3.0 (1.0. 7.0) |
ICU, intensive care unit; HD, hemodialysis; PD, peritoneal dialysis; RRT, renal replacement therapy.
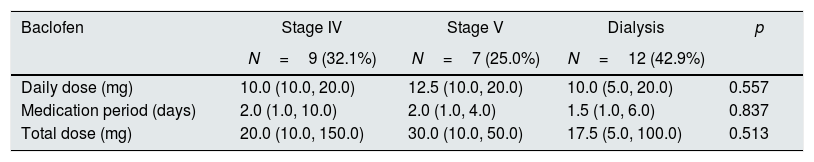
Of the patients with baclofen neurotoxicity, the lowest total dose was 5mg in patients who received peritoneal dialysis (Table 5, #28) and the highest total dose was 150mg in CKD stage 4 patients (Table 5, #6). The minimum daily dose for baclofen neurotoxicity was 10mg, 10mg, and 5mg, respectively, in patients with CKD stage 4, non-dialysis stage 5, and on-dialysis patients (Tables 6 and 7).
Baclofen dose according to the CKD stages in the patients with neurotoxicity.
| Baclofen | Stage IV | Stage V | Dialysis | p |
|---|---|---|---|---|
| N=9 (32.1%) | N=7 (25.0%) | N=12 (42.9%) | ||
| Daily dose (mg) | 10.0 (10.0, 20.0) | 12.5 (10.0, 20.0) | 10.0 (5.0, 20.0) | 0.557 |
| Medication period (days) | 2.0 (1.0, 10.0) | 2.0 (1.0, 4.0) | 1.5 (1.0, 6.0) | 0.837 |
| Total dose (mg) | 20.0 (10.0, 150.0) | 30.0 (10.0, 50.0) | 17.5 (5.0, 100.0) | 0.513 |
Patients with baclofen neurotoxicity were divided into CKD stage 4, non-dialysis stage 5, and on-dialysis patients, and there was no significant difference between the three groups. The daily dosage was 10.0mg (10.0, 20.0), 12.5mg (10.0, 20.0), and 10.0mg (5.0, 20.0), and the total dosage was 20.0mg (10.0, 150.0), 30.0mg (10.0, 50.0), and 17.5mg (5.0, 100.0), respectively (Table 7).
DiscussionTo our knowledge, this is the first study regarding the prevalence of baclofen neurotoxicity in patients with severe CKD. In this study, among 401 patients with severe CKD, 28 (7.0%) patients had baclofen neurotoxicity. However, there are, in fact, a number of other cases that have not been reported in the medical records because they are not as severe as compared with these patients. The baclofen neurotoxicity is not well known; there is no clearly defined dose reduction criterion, and there is a high likelihood of iatrogenic neurotoxicity. According to the results of this study, baclofen should be used with caution when the serum albumin level is low.
This is also the first study showing that hypoalbuminemia is the independent risk factor for baclofen neurotoxicity. About 30–35% of baclofen binds to protein, and what is actually working is free baclofen that does not bind to protein.17 For this reason, the lower the serum albumin, the higher the concentration of baclofen and the greater the likelihood of neurotoxicity. However, the study by Benet and Hoener28 showed that the level of protein-binding in plasma does not, in fact, affect patients; so, there is no need to adjust the concentration. Therefore, pharmacokinetic studies on baclofen, plasma protein, and albumin are necessary to define the exact mechanism. The concentration of baclofen can be measured using a gas chromatography/mass spectrometry or high-performance liquid chromatography on a blood sample, but the results are delayed and unusable as a toxicological screening test.1,14 In the future, good tests for monitoring baclofen dosage should be developed.
Because many other risk factors cause baclofen neurotoxicity besides renal function, it is difficult to precisely define the degree of renal dysfunction that might lead to contraindicate the use of baclofen medication.18 Su et al.4 and El-Husseini et al.1 recommended not using baclofen when the eGFR was <30mL/min/1.73m2 and recommended lower doses and longer intervals when the eGFR was between 30mL/min/1.73m2 and 60mL/min/1.73m2. Roberts et al.3 recommended that baclofen be administered at the lowest dose, according to the manufacturer's guidelines, in patients with mild CKD (eGFR >60mL/min/1.73m2). For patients with moderate or severe CKD (eGFR <60mL/min/1.73m2), baclofen is avoided. Vlovonou et al.5 suggested that baclofen daily doses should be reduced by 1/3, 1/2, and 2/3, respectively, because in mild, moderate, and severe CKD, baclofen removal rates are reduced by 34%, 49%, and 64%. Hadjiyannacos et al.25 recommended that patients on dialysis should not be given >5mg baclofen per day. According to Wolf et al.,29 when baclofen is difficult to use due to decreased kidney function, it may be replaced by cyclobenzaprine, tizanidine, and dantrolene for muscle spasm.
When patients with baclofen neurotoxicity were divided into CKD stage 4, non-dialysis stage 5, and on-dialysis patients, the median values of daily doses were 10.0mg, 12.5mg, and 10.0mg, respectively, and the minimum daily dosage was 10.0mg, 10.0mg, and 5.0mg, respectively. The median doses of medication up to symptom onset were 20.0mg, 30.0mg, and 17.5mg, respectively (Table 7). Vlovonou et al.5 recommended a baclofen dose of 2.5mg q 12h for severe CKD <30mL/min/1.73m2eGFR. However, according to the results of this study, it may be possible to use up to 7.5mg in patients with CKD stages 4 and 5 (non-dialysis), and 2.5mg in dialysis patients.
In the case of diabetic nephropathy, which showed significant results when analyzed using a chi-squared test for neurotoxicity according to the underlying disease of the patient, a multiple logistic regression analysis showed no significant results (Tables 2 and 4). According to the American Diabetes Association, diabetic nephropathy is a clinical diagnosis made based on the presence of albuminuria and/or reduced eGFR in the absence of signs or symptoms of other primary causes of kidney damage.30 The stage of albuminuria is divided by measuring the albumin-creatinine ratio, which is defined as normal <30, microalbuminuria=30–299, and overt proteinuria ≥300 (mg/g creatinine). Moreover, >0.5g of proteinuria in 24h occurs in 15–40% of people with type 1 diabetes and 5–20% of people with type 2 diabetes.31 Although the amount of total proteinuria in patients with diabetic nephropathy is typically subnephrotic (<3.5g/24h), in some earlier studies of patients with type 1 and type 2 diabetes and nephropathy, as many as 70–80% patients had nephrotic range proteinuria.32 In patients with diabetic nephropathy, severe proteinuria leads to hypoalbuminemia, and hypoalbuminemia is a risk factor for baclofen neurotoxicity. For this reason, a multiple logistic regression analysis that controlled the influence of serum albumin could be interpreted as showing no significant results in diabetic nephropathy.
The male population was overwhelmingly large in the entire population receiving baclofen, but the interpretation is not clear. There are reports that cisplatin-induced hiccups are common in men (97.4%).33 In Cymet's study of hiccups,34 91% of the population was male, but the reason was unclear. In many other studies, the majority of people with hiccups were males.35,36 There is a reason why hiccups are more likely to occur in men, but it is not yet clear. In this study, 93.0% (373) of the patients received baclofen because of hiccups, which seems to account for the male majority (Table 1).
The total dose was significantly lower in the neurotoxic group (p=0.041) (Table 2), which was perceived to be due to the early drug withdrawal in patients with neurotoxicity. In the neurotoxic group, the median dose of daily baclofen was 10.6mg (Table 2). In the study by El-Husseini et al.,1 the mean value of the daily dose was 20mg, which was different from that in this study. The median time to symptom onset after baclofen treatment was 39.5h, which was shorter than the outcome found by El-Husseini et al.1 (Table 6).
This study has potential limitations. First, the major limitation of this study is its unicentric, retrospective, and observational nature, which may have inherent selection bias including controlling the dosage of baclofen or the characteristics of the patient. However, because of ethical issues, prospective research including dose control may be difficult in the future. Second, it is impossible to completely exclude the history of CNS diseases and the effects of other drugs that affect the CNS. Although CNS disease and CNS drugs were found to be unrelated to neurotoxicity (Table 2), 26 out of 28 baclofen neurotoxic patients took drugs that affect the CNS (Table 5). CNS drugs can be a confusing variable, so this requires careful attention to interpretation.
ConclusionsThe prevalence of baclofen-induced neurotoxicity in patients with severe CKD was 7.0% (28 of 401 patients). Serum albumin is an independent risk factor for neurotoxicity, and baclofen should be used cautiously in patients with hypoalbuminemia. In the studied population, it was found that a lower baclofen dose such as 7.5mg in patients with CKD stages 4 and 5 (non-dialysis) and 2.5mg in patients who receive dialysis was associated with the presence of fewer adverse effects.
Conflicts of interestThe authors have no conflicts of interest to declare.