Familial Mediterranean fever (FMF) is a clinically well known and the most frequent monogenic auto-inflammatory disease affecting mostly people who have a Mediterranean descent. Typical self-limited attacks of fever, abdominal pain, polyserositis and also an imminent complication, amyloidosis, might be hard to manage in the clinical setting. Two decades earlier, mutations of MEFV, pyrin innate immunity regulator gene (MEFV) located on chromosome 16 were accused of being responsible of FMF; from that time on, lots of mutations have been described, but some of them were not associated with a phenotype of the disease and named ‘variants of uncertain significance.1
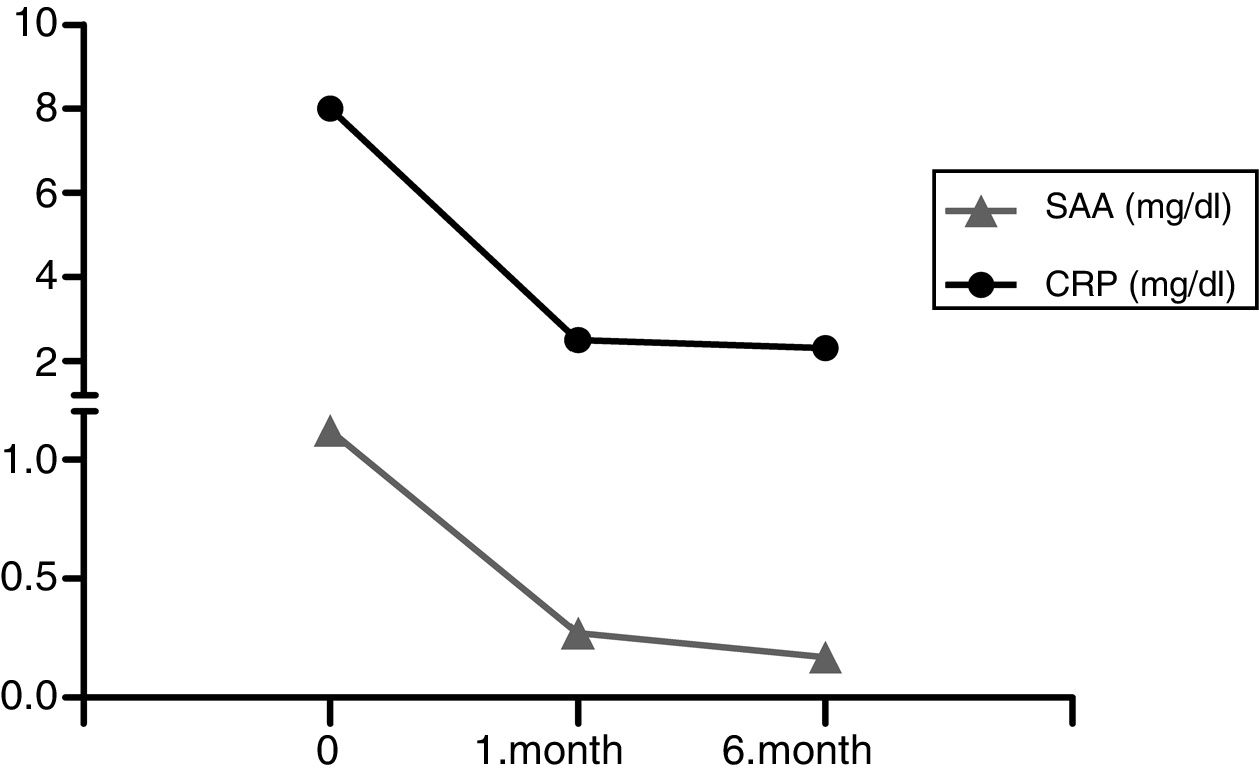
A 24-year-old female Turkish patient started peritoneal dialysis in our unit after 10-years clinical diagnosis of FMF and amyloidosis in May 2008. She was homozygous both for MEFV R202Q and M694V. After eight years on PD without any complications except a few attacks of peritonitis, she had kidney transplantation from a deceased donor with anti-thymocyte globulin as an induction immunosuppression. She discharged from the hospital when she achieved normal graft function with triple immunosuppression consisting steroid, tacrolimus, mycophenolate besides 2-mg/day dose of colchicine. After one month of discharge, she admitted to the hospital with fatigue and diarrhea, severe and treatment-resistant hyponatremia was found in her laboratory findings. Therefore first we tapered colchicine dose and changed tacrolimus to everolimus. Then we changed mycophenolate to mycophenolate sodium and had to taper dose of colchicine to 0.5mg/day. After diarrhea and hyponatremia settled, the patient had fever, abdominal discomfort and chest pain which she had already experienced before PD and kidney transplantation. Acute kidney injury with serum creatinine level of 2.3mg/dl (serum creatinine level was 1.1mg/dl at the time of discharge) and increases in acute phase reactants (serum C-reactive protein (CRP) level: 9mg/dl, normal range: <5mg/dl) occurred without any signs or symptoms of infection at the same time. Circumferential 1-cm pericardial effusion, thickened mesenteric folds, minimal free fluid in Douglas’ pouch were found in echocardiographic and ultrasonographic evaluations, excluding the possible diagnosis of sclerosing encapsulated peritonitis and early surgical complications such as lymphocele. But position and volume of free fluid in the abdomen were not appropriate for sampling, and there were no signs of cardiac tamponade. She did not tolerate higher doses of colchicine because of gastrointestinal adverse effects. After attack dissolved with conservative treatment, CRP was still elevated but graft function turned to normal with serum creatinine level 1.07mg/dl. Proteinuria was not detected before or during the attack. We added anakinra (Kineret, Sobi Inc, Stockholm, Sweden) (subcutaneously, 100mg/day) to colchicum treatment because of intolerance to colchicine and continuous inflammatory state. In the first month of anakinra, serum amyloid A decreased from 1.12mg/dl to 0.27mg/dl (normal range <0.5mg/dl) and serum CRP level from 8mg/l to 2.49mg/l shown in Fig. 1. Pericardial effusion decreased from 1cm to 0.3cm and free fluid in Douglas’ pouch disappeared. She had never experienced any other attack and anakinra was well tolerated with only minimal local irritation resolving with changing injection sites through the sixth month of treatment.
The relationship between mutation types and disease characteristics are well known in some mutations such as a severe disease is associated with M694V but R202Q has controversial information in the literature.2 Cekin et al. found that R202Q is classified as the most common polymorphism in a Turkish cohort and Yigit et al. reported that homozygous R202Q mutation was significantly higher in patients with FMF than control group.2,3 A study with a Turkish children cohort revealed that recurrent abdominal pain were commonly seen in patients with M694V and R202Q mutations. But as observed in this patient, chest pain was commonly seen with the R202Q mutation.4 Therefore R202Q might not be a disease-causing mutation but may be a mutation that increases the severity of the disease.
Colchicine is still the principal treatment but loses its efficacy although with the maximum therapeutic dose of 3mg/day for adults in some patients who are generally called ‘colchicine-resistant’ or ‘colchicine-refractory’.5 Hopefully, anti-IL-1 therapies including anakinra, canakinumab, and rilonacept, have been an alternative strategy for resistant or refractory patients.1 Recently, a randomized controlled study has shown that anakinra is a safe and effective option for patients with resistant FMF.6 Although few cases were reported about treatments with anti-IL-1 agents after kidney transplantation in patients with FMF, there is still a lack of information in those patient group.7–9
We recommend that all patients with FMF should be genetically tested and R202Q may be taken into account to assess disease severity. And also even after kidney transplantation, anakinra as a sufficient and well-tolerated agent can be added to colchicine treatment in resistant, refractory or intolerant patients.