INTRODUCTION
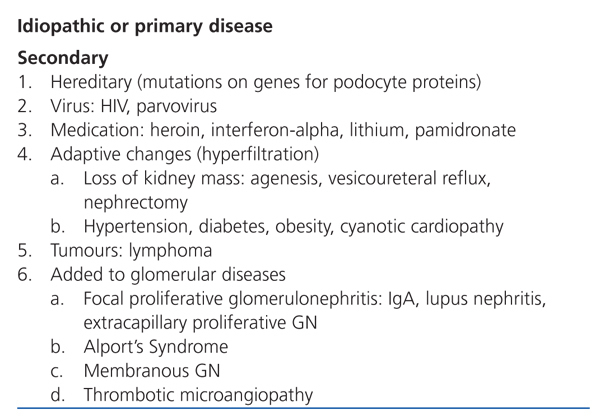
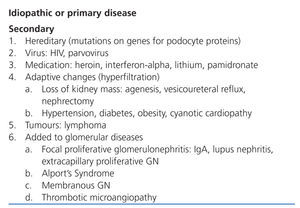
Focal segmental glomerulosclerosis (FSGS) is defined as an increase in the mesangial matrix in some glomeruli with obliteration of capillary lumens, sclerosis, hyalinosis, foam cells, and adhesions to the Bowman’s capsule. The damage is non-specific, and several different causes have been described. As such, it is essential to distinguish between idiopathic and secondary forms of the disease (Table 1), since their pathogenesis, prognosis, and treatment are very different.1
Fortunately, FSGS is an uncommon disease that is found only in 9% of all kidney biopsies in our country, with stable rates in recent years. However, it is the third-leading cause of nephrotic syndrome, preceded only by membranous nephropathy and minimal change nephropathy.2 Here, we will only be referring to idiopathic FSGS that presents with nephrotic syndrome, since this is a major challenge for the attending physician given the knowledge gaps regarding its pathogenesis. As with other glomerular diseases, its treatment must be based on the best evidence available through controlled clinical trials. However, few studies have been performed to resolve the current problems with treatment, and many of them are of poor quality, as reported by Quereda and Ballarín in an excellent systematic review published in Nefrología in 2007.3 Their conclusions have not lost their relevance and coincide with the recommendations soon to be published in the KDIGO guidelines for the treatment of glomerulonephritis. FSGS is no exception to this rule of low number and quality of clinical trials studying glomerular diseases, with even lower representation than lupus nephritis and membranous nephropathy.4
INITIAL TREATMENT
Patients with idiopathic FSGS that do not develop a nephrotic syndrome, as well as those with secondary FSGS, do not receive immunosuppressive treatment (recommendation 1C, GRADE system). In these cases, the standard treatment consists of: 1) maintain blood pressure below 130/80mm Hg; 2) administer angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, or both; 3) administer statins; 4) administer antiplatelet or anticoagulation therapy; and 5) diet for renal protection.5 The initial treatment is based on a prednisone cycle (1mg/kg/day, maximum of 80mg/day or 2mg/kg/day on alternate days, maximum of 120 mg), which should be maintained for 16 weeks before the condition is declared corticosteroid-resistant.6 Treatment with prednisone can cause remission of the disease in 60%-70% of patients, according to the studies. These responses depend on the intensity of the initial proteinuria, the tubular/interstitial lesions, and the baseline creatinine level, although it is not possible a priori to separate the patients that will respond to corticosteroid treatment from those that will not. As a rule, this should not be combined with treatment using other immunosuppressive drugs, unless there is a risk of toxicity or intolerance to steroids (obesity, osteoporosis, diabetes, advanced age, or psychiatric imbalances). If they were to be used, calcineurin inhibitors are recommended (suggestion 2D), as will be indicated later when discussing corticosteroid-resistant forms of the disease.3 Partial or complete remission of proteinuria has been associated with a good prognosis, and it is the most indicative marker of patient evolution, even more than clinical and histological results.7 Approximately 30%-40% of patients are corticosteroid-resistant, presenting problems for developing a treatment plan, since resistance to corticosteroids is the strongest predictor for the development of chronic kidney disease: 35% at 5 years and 70% at 10 years. As such, the current conundrum is: how can we treat idiopathic FSGS that has not responded to steroids or is corticosteroid-dependent? Can we modify the patient evolution towards kidney failure? Here, we will go through the different treatments recommended in corticosteroid-resistant FSGS according to the evidence published on the subject (Table 2). Obviously, each case should be analysed individually, and treated according to the personal opinion and experience of the physicians that directly attend to each patient.
CALCINEURIN INHIBITORS
Cyclosporin A (CsA) is the best documented method of treatment in controlled trials and observational studies.3,8 The recommended dose is 2-5mg/kg/day administered in two doses (with levels of 125-175ng/ml) during a minimum of 6 months. If at the end of this time there is no response, treatment must be suspended and the patient should be considered resistant to CsA. In the case of a partial or complete response, which appears in 60%-70% of cases,9,10 treatment should be maintained for at least 12 months, and can then be progressively reduced by 25% every 2 months. Approximately half of all patients that initially respond to CsA develop resistance eventually. Fewer studies exist regarding the use of tacrolimus in the treatment of corticosteroid-resistant FSGS, but Segarra et al11 obtained positive results from using tacrolimus (0.15mg/kg/day; levels of 5-10ng/l) and low-dose steroids (0.15mg/kg/day) in 25 patients with previous resistance to steroids and CsA. Therefore, its use in patients that have not responded to CsA is an attractive treatment option that deserves a try before moving on to other alternatives. Even so, treatment with calcineurin inhibitors creates other problems: nephrotoxicity and recurrence after treatment suspension. The first condition can be avoided by using the minimum possible dose, monitoring patient levels, and by performing a kidney biopsy in order to evaluate vascular and interstitial damage. Recurrence is very common after reducing the dosage or suspending treatment with this drug, reaching even 70% in some studies. A study, sponsored by the GLOSEN (glomerulonephritis study group of the Spanish Society of Nephrology), has been recently performed on 5 patients, who were administered calcineurin inhibitors as an induction therapy, followed by rituximab once remission was established, with no positive results in avoiding recurrence after treatment suspension.12 Recurrence is still an unresolved problem that requires more in-depth studies. On the other hand, given that approximately 30% of cases do not respond to calcineurin inhibitors, other treatments have also been tested.
MYCOPHENOLATE MOFETIL
Experience with this drug is scarce,6 with an approximately 44% of cases showing a partial response, but half of these patients suffer a recurrence when medication is suspended.7 This is an option when other immunosuppressive drugs cannot be administered, or when steroid dosage must be decreased (suggestion 2C).
RITUXIMAB
In a recent study that was also sponsored by GLOSEN,13 involving 8 patients with FSGS resistant to other drug treatments, rituximab had a positive effect in only 3 cases, making it an unattractive alternative, at least as a monotherapy. Therefore, controlled studies are needed,8 but it still can provide an alternative when nephrotoxicity develops due to the administration of calcineurin inhibitors.14
ALKYLATING AGENTS
Some experience has been gained in the administration of cyclophosphamide and chlorambucil, as they were used before the advent of calcineurin inhibitors. However, they only produce a complete response in 20% of cases, and a partial response in 45%. These results, along with the adverse effects (infections, tumours, leukopenia, infertility), have severely reduced the role of these drugs,9 with insufficient evidence to support their use.6,15,16
COMBINED TREATMENTS
Given the limitations presented by the previous drug treatments, some authors have incorporated the concept of synergistic effects of immunosuppressive drugs (taken from the experience gained in transplantations) into the treatment of glomerular diseases. In this issue of Nefrología, Segarra et al17 have published on their experience in treating primary FSGS in 27 adult patients with previously failed treatment plans using steroids and CsA, combining CsA (4mg/kg/day) with mycophenolate mofetil (2000mg/day) for 12 months. This was an observational study with no controls, and it produced disappointing results. None of the patients in this study reached complete remission of the disease, and only one fourth partial remission. Additionally, the glomerular filtration rate was significantly reduced in all patients, and 59% of cases developed uraemia at the end of the 5-year follow-up period, which is a similar result to that achieved by treating patients symptomatically. Lastly, the authors used a multivariate analysis to show that initial glomerular filtration rates and mean proteinuria during the follow-up period were correlated with renal impairment. El-Reshaid et al18 had already tested a similar combined treatment, with somewhat better results, but in patients without previous failure of CsA treatment. The role that this three-part association may play in the treatment of idiopathic FSGS resistant to steroids and CsA is still unclear. Although the authors did not perform a genetic analysis, it is quite probable that the hereditary forms of the disease were not present in the study, since the necessary genetic mutations (NPHS1, NPHS2, alpha-actinin-4, CD2P, TRPC-6 and others) are very rare in adults.19 With these results, the triple therapy using steroids, CsA, and mycophenolate mofetil appears not to decrease proteinuria or slow the development of kidney disease.
Although the treatment of corticosteroid-resistant idiopathic FSGS is still an unresolved issue, we must not be pessimistic. Several different clinical trials have been initiated20,21 that may help to improve the current results. As the KDIGO guidelines will state, more controlled clinical trials are needed to compare the efficacy of calcineurin inhibitors, mycophenolate mofetil, rituximab, and alkylating agents in treating corticosteroid-resistant FSGS. Lastly, we must re-evaluate the use of our current arsenal of immunosuppressive drugs, whose mechanisms of action are still unknown and are mostly empirical.22 According to a recent review by Meyrier,23 the factor or factors that lead to an increase in capillary permeability must be identified in order to treat these patients accordingly.
In conclusion, the treatment of idiopathic FSGS patients with nephrotic syndrome is still unresolved, and clinicians are faced with a challenge in producing some type of response in the patient and slowing or halting the evolution towards kidney failure. In spite of the negative results obtained by the excellent study by Segarra et al,17 which inspired this “Editorial Comment,” we must continue to research ways to improve the current unsatisfactory results.
KEY CONCEPTS
1. FSGS is a non-specific glomerular lesion that is classified as idiopathic (primary) or secondary.
2. Patients with idiopathic FSGS that do not develop nephrotic syndrome and cases of secondary FSGS should not be treated using steroids or immunosuppressive drugs.
3. Idiopathic FSGS patients with nephrotic syndrome should be initially treated with a cycle of steroids (or steroids and CsA in the case of intolerance to high levels of steroids) for a minimum of 4 weeks and a maximum of 16 weeks.
4. Patients with corticosteroid-resistant FSGS should be treated using CsA and low doses of steroids for at least 6 months, monitoring blood levels and nephrotoxicity. Patients that do not respond to treatment can benefit from the administration of tacrolimus.
5. Mycophenolate mofetil and rituximab can be effective alternatives in the case of resistance to CsA, although little evidence exists.
6. Alkylating agents (cyclophosphamide and chlorambucil) are only barely indicated and their use is not recommended, except for in very severe cases of dependence on corticosteroids.
7. Until now, combined treatments have not produced any positive results.
8. New treatments are needed based on controlled clinical trials that can induce a response in proteinuria and avoid the evolution of the disease towards kidney failure.
Table 1. Causes of focal segmental glomerulosclerosis
Table 2. Treatments for idiopathic focal segmental glomerulosclerosis patients with nephrotic syndrome