To the Editor,
Cyclophosphamide is a synthetic alkylating agent used in chemotherapy and as an immunosuppressive agent. Among its adverse effects are infections, myelosuppression, haemorrhagic cystitis, hypersensitivity reactions, digestive/hepatic, pulmonary, cardiac and neurological toxicity, sterility and syndrome of inappropriate antidiuretic hormone hypersecretion (SIADH).1-3
We describe a patient with abdominal pain and an increase in hepatic and pancreatic enzymes after cyclophosphamide administration.
Male, 57-year-old patient, admitted for renal failure. Patient history: arthralgia and arthritis during the past 10 years, deafness, frequent nosebleeds; pancreatitis due to cholelithiasis a month before. Physical examination: he did not have fever, blood pressure: 130/70mm Hg; lung crackles in both lungs, and remaining data were normal. The analysis showed: haemoglobin: 8.1g/dl: (13-17); creatinine: 6.44mg/dl (0.84-1.25); urea: 163mg/dl (17-43); albumin: 2.63g/dl (3.50-5.20); GOT: 5IU/l (10-39); GPT: 15IU/l (10-45); GGT: 71IU/l (10-55); alkaline phosphatase: 102IU/l (30-120); total bilirubin: 0.69mg/dl (0.3-1.2); amylase: 100IU/l (22-80); sediment: 60-100 red blood cells per field; proteinuria: 1.5g/24hr; negative urine culture. Negative c-ANCA, positive p-ANCA, negative PR3, MPO: 50.5(N<7IU/ml); negative anti-MBG antibodies, negative serology tests for hepatitis B, C and human immunodeficiency virus (HIV). Abdominal ultrasound: cholelithiasis, normal-size kidneys; pulmonary computerised tomography (CT) revealed bilateral nodes. The kidney biopsy showed focal and segmental necrotising glomerulonephritis with few deposits seen in the immunofluorescence. The patient was diagnosed with ANCA-positive vasculitis (microscopic polyangiitis/Wegener’s granulomatosis).
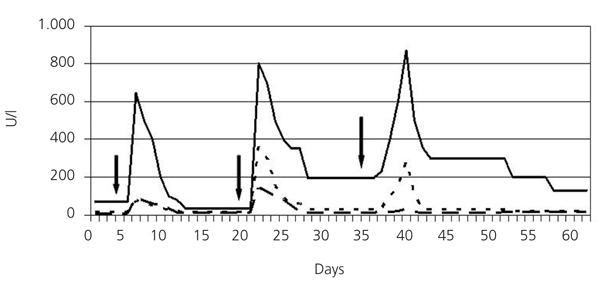
He was treated with three 500-mg boli of methylprednisolone, continuing with 60mg of prednisone a day orally, haemodialysis and plasmapheresis. He also received omeprazole, calcium carbonate, sevelamer, furosemide and erythropoietin. The first cyclophosphamide bolus was administered (500mg) and 12 hours later the patient presented with sweating and diffuse abdominal pain, with no signs of peritoneal irritation. He presented with GOT: 90IU/l; GPT: 76IU/l; GGT: 645IU/l; alkaline phosphatase: 109IU/l; total bilirubin: 0.66mg/dl and amylase: 173IU/l. These parameters normalised in the following days. Fifteen days later the second cyclophosphamide bolus was prescribed (750mg), and 24 hours later the pain in the right hypochondrium reappeared. He presented with GOT: 144IU/l; GPT: 358IU/l; GGT: 802IU/l; alkaline phosphatase: 103IU/l; total bilirubin: 6.44mg/dl; amylase: 208IU/l; lipase: 378IU/l (21-67); which decreased in the following days. The magnetic resonance cholangiography showed cholelithiasis with no signs of complications, bile duct not dilated, free of choledocholithiasis. After 15 days he was administered 50mg/day of oral cyclophosphamide. After 4 days he presented with self-limited pain in the right hypochondrium. He had GOT: 27IU/l; GPT: 281IU/l; GGT: 871IU/l; alkaline phosphatase: 129IU/l; total bilirubin: 0.74mg/dl; amylase 97IU/l and lipase 154IU/l (Figure 1). Cyclophosphamide was withdrawn and treatment with mycophenolate mofetil was started at a dose of 500mg/8 hours. After a month, the patient was asymptomatic, normotensive, with spontaneous diuresis of 2l/day, Cr 2.59mg/dl; GPT: 9IU/l; GPT: 28IU/l; GGT: 155IU/l; alkaline phosphatase: 105IU/l; total bilirubin: 0.68mg/dl; normal amylase and lipase, weakly positive MPO-ANCA. The lung computerised tomography (CT) showed that the images had almost disappeared.
The effectiveness and toxicity of cyclophosphamide differs greatly from one patient to another, which has mainly been related to pharmacokinetic and pharmacogenetic mechanisms.1,2,4,5 As such, Navin Pinto et al suggest that certain polymorphisms of enzymes metabolising cyclophosphamide (cytochrome P450, glutathione S-transferases and aldehyde-dehydrogenases) could be related to this variation.3 A relationship has been found between high doses of cyclophosphamide and its toxic metabolites (acrolein and phosphoramide mustard) and hepatotoxicity.2,4 Hepatotoxicity has also been related to high levels of other metabolites such as 4-hydroxycyclophosphamide2 and o-carboxyethyl-phosphoramide mustard.4 Furthermore, it has been shown that cyclophosphamide-induced adverse reactions could be due to cholinesterase inhibition.6 Cyclophosphamide-induced hepatotoxicity is characterised by cytolysis and cholestasis, as occurred in this case. It may appear with oral or intravenous administration and seems to depend on the dosage. Three histological patterns have been described: massive hepatic necrosis, necrosis of perivenous hepatocytes and diffuse hepatocellular damage with mild steatosis.1
Patients with advanced renal failure can have high levels of amylase (up to three times the upper normal limit) and lipase (up to double). In this patient, after the second cyclophosphamide bolus, an increase in lipase, five times the normal level was observed, which may indicate pancreatic involvement. As far as we are aware, cyclophosphamide is not associated with pancreatitis, but iphosphamide, another nitrogen mustard, similar to cyclophosphamide is described to produce pancreatitis.7
ANCA vasculitis can affect the digestive system. Our patient’s cholelithiasis was not complicated; biliary colic could cause analytical alterations similar to those that developed, but in the magnetic resonance cholangiography there were no signs of choledocholithiasis. In this case, there was a clear temporary relationship with cyclophosphamide, which supports the drug’s role. According to Naranjo et al’s8 scale, hepatotoxicity and cyclophosphamide are likely to be related in this case.
This case shows that hepatic and pancreatic functions must be monitored during treatment.
Figure 1. Evolution of GOT, GPT and GGT during cyclophosphamide treatment