Hepatitis C virus (HCV) infection is a factor that reduces the survival of the patient and the graft in renal transplant (RT). The availability of directly acting antivirals agents (DAAs), very effective and with an excellent safety profile, it allows eradicate HCV from patients with kidney disease, and this is a revolutionary radical change in the natural evolution of this infection, until now without effective and safe treatment for the contraindication use of interferon in kidney transplant patients.
The efficiency of some DAAs for all genotypes, even in patients with renal insufficiency constitutes a huge contribution to eradicate HCV in the RT population independently the genotype, severity of kidney failure, progression of liver disease and previous anti HCV therapy.
All this is raising, although with controversies, the possibility of use kidneys from infected HCV+ donors for transplant in uninfected receptors and can be treated successfully in the early post-TR, thus increasing the total “pool” of kidneys for RT.
La infección por el virus de la hepatitis C (VHC) es considerada un factor que reduce la supervivencia del paciente y del injerto en trasplante renal (TR). La disponibilidad de los nuevos antivirales de acción directa (AAD), muy eficaces y con un excelente perfil de seguridad, está permitiendo erradicar el VHC de los pacientes con enfermedad renal, y ello supone un revolucionario cambio radical en la evolución natural de esta infección, hasta ahora sin un tratamiento eficaz y seguro por la contraindicación del interferón en los trasplantados renales.
La eficacia de algunos AAD para todos los genotipos, incluso en pacientes con insuficiencia renal, constituye una enorme contribución para erradicar el VHC en la población con TR al margen del genotipo, del grado de insuficiencia renal, de la progresión de la hepatopatía y de la existencia previa de tratamiento anti-VHC.
Todo ello está planteando, aunque con controversias, la posibilidad de utilizar riñones de donantes infectados VHC+ para trasplante en receptores no infectados y poder tratarse y curarse tras el TR precoz, aumentando así el pool total de riñones para TR.
Hepatitis C virus (HCV) infection is the main cause of liver disease in kidney transplant patients (RT) with increased risk of chronic liver disease, cirrhosis and hepatocarcinoma.1–5 In addition, HCV infection is an independent risk factor for kidney graft loss and patient death.1,2,5 However, the survival of HCV+ patients is significantly higher in transplanted patients than HCV+ patients on the waiting list.1 Therefore, kidney transplant is the best stategy for HCV RNA+ patients with end-stage renal diseae (ESRD). Also, RT in HCV+ patients receiving kidneys from HCV+ donors has been proven to be effective and safe at the long term.5
Since 2014, the new antivirals with direct action (DAA) with an interferon-free (INF) scheme have achieved cure of the infection in almost 100% of cases, so HCV eradication is changing the natural history of this infection.6–8 Therefore, it is now pertinent to review the problem of HCV infection in RT and describe the most outstanding and current data of DAAs.
Here, we review the relationship between HCV infection and RT, the management of patients on dialysis and after transplantation during the INF era, the impact of DAAs on patients in the waiting list and with RT, and also the new possibilities of RT using kidneys donor that are HCV+.
Hepatitis C and renal transplantationImpact of renal transplantation on HCV infectionHepatic disease due to HCV infectionRT HCV+ patients have an increased risk of progression to chronic liver disease, and although this process is usually slow and does not occur in all patients,1,9–14 there is an increased risk of cirrhosis and hepatocarcinoma as compared to RT patients without HCV.
Except for Fibrosing cholestatic hepatitis in 1.5% of cases15 the initial course of HCV infection after transplantation is benign; the biochemical and histological changes are observed after a long period of evolution.1
It is known that the immunosuppression received after RT is associated with an increase in viral replication and progression of liver fibrosis. In a retrospective study, the annual progression of necrosis, inflammatory activity and liver fibrosis evaluated by sequential liver biopsies (period of 7.1±4.0 years) in 28 RT HCV+ patients was significantly greater than in 28 immunocompetent HCV+ patients.10 During the follow-up, the evolution to cirrhosis was 21.4% in the HCV+ RT patients compared to 3.6% in the immunocompetent group, while liver mortality was significantly higher in the HCV+ RT patients (10 vs 0%, p<0.05).10
Factors associated to the development of chronic liver disease are: the severity of liver disease at the time of transplantation, coinfection with hepatitis B virus (HBV), prolonged time of after transplantation and the use of antilymphocyte.1,3,16–18
Influence of hepatitis C on renal transplantationAcute rejectionReports on the risk of acute rejection in HCV+ RT patients are controversial.1 Our group has reported that HCV infection induces a state of immunodeficiency based on a reduction in the percent of T lymphocytes helper and an alteration in the proliferative response of T lymphocytesto mitogens.19 These abnormalities may explain a decreased incidence of acute rejection described by some authors. Thus, Roth et al.14 have reported a 14.5% incidence of acute rejection in RT HCV+ patients followed for 20 years. In a Spanish study, the rate of acute rejection in RT HCV+ patients was higher during the 1998–2002 period than during 1990–1994 (25% vs. 20.6%, p=0.01), probably due to the fact that during the last period there was a greater number of re-transplants and hyperimmunized patients.19
Secondary infectionsRT HCV+ patients have an increased incidence of postoperative infections and more frequent severe infections of the central nervous system, lungs, and bacteremia, than the RT HCV− patients.1,2,20 In fact, infection is the second cause of death in these patients, more than liver disease.21 A Spanish multicenter prospective study conducted between 2003 and 2005 found no difference in the average incidence of infection between RT HCV+ versus RT HCV−, although HCV+ recipients had a higher incidence of bacteremia and urinary tract infections. In this analysis, the presence of HCV infection was an independent risk factor only for bacteremia.22 And, in a retrospective case–control study, our group demonstrated that HCV infection is an independent risk factor for the development of tuberculosis in RT patients.23
Post-transplant extrahepatic neoplasmsThe Australian and New Zealand registry has reported that neoplasms represent the second cause of death in HCV+ patients, ahead of liver related causes.24 Although this data has to be confirmed by additional studies, these findings suggest that HCV infection may play a role in the pathogenesis of post-transplant proliferative disease and other hematological diseases, such as multiple myeloma.25–28 Also, a direct effect of HCV infection has been demonstrated in the carcinogenesis of lymphoid cells,29 as well as the control of Hodgkin lymphoma after antiviral treatment.30 Therefore, close monitoring is recommended to rule out neoplasms in RT HCV+ patients.
De novo diabetes in renal transplantation (NDRT)In a meta-analysis that included 30,099 patients with RT, there was a marked increase in the risk of NDRT in HCV+ patients.30 In addition, administration of tacrolimus in HCV+ patients is associated with an increased risk of NDRT.31
Insulin resistance due to the inhibitory action of the virus in the regulatory pathways in the liver seems to be the most accepted pathogenic mechanism to explain the relationship between HCV infection and NDRT.
From a practical point of view, the most rational attitude may be to decrease the doses of tacrolimus or to apply strategies to eliminate steroids. In any case, the use of drugs that favor NDRT should be balanced to optimize the anti-rejection treatment and minimize the risk of hyperglycemia.1
Kidney disease induced by HCV infectionRecurrent and de novo glomerulonephritisThere have been described Glomerular lesions in patients with HCV infection with either native kidneys or transplanted kidneys.32,33 After RT, it has been described membranoproliferative glomerulonephritis (MPGN) recurrent or de novo either Cryoglobulinemic or non-Cryoglobulinemic, membranous glomerulonephritis (MGN), post transplantation acute glomerulopathy, thrombotic microangiopathy and transplant glomerulopathy (TGN).34–37 In fact, HCV infection is a factor that predicts the development of proteinuria in RT patients.38 The most frequent GN is the GNMP, usually recurrent after a re-transplant, followed by the GNM.32,34
The pathogenesis of MPGN and MGN is based on the glomerular deposition of immunocomplexes containing viral RNA, paradoxically in immunosuppressed patients.32,33
Transplant glomerulopathyA study from a group of Boston published results of 25 patients diagnosed of TGN by optical microscopy, showing that there are three overlapping etiologies: the acute humoral rejection, hepatitis C and thrombotic microangiopathy. These authors interpreted that TGN is a pattern of histological lesion with different prognosis and therapeutic implications.39 Therefore, GNT is not synonymous with chronic humoral rejection; other etiologies such as HCV infection may explain these lesions. In fact, our group has shown that HCV infection is an independent risk factor for TGN (diagnosed by electron microscopy).40 In addition, graft loss occurs more quickly in patients with TGN and HCV+ than TGN and HCV−. Whether this is explained by increased immune risk in HCV+ patients or by the direct effect of the virus on the glomeruli will need to be investigated
Impact of HCV infection on survivalPatient survivalDespite the worse prognosis of patients with active HCV infection, RT offers survival advantages over those HCV+ patients on the waiting list for RT that remain on regular dialysis.41–45
However, despite the advantages of RT compared to dialysis, the long-term survival of the HCV+ patient after RT is significantly reduced as compared with HCV− RT patients.1,46–51 A Spanish study has reported a 10 year survival of 77.5% in HCV+ patients vs 84.5% in HCV−.46 In two meta-analyzes, Fabrizi et al.52 and Rostami et al.53 clearly demonstrated that RT HCV+ patients have an increased risk of death. Consistent with previous reviews, all causes of mortality were significantly higher in HCV+ patients, being cardiovascular disease the most frequent cause of mortality. In fact, HCV infection was a risk factor for the development of atherosclerosis.54 The other causes of death were, in order of frequency, infections, tumors and liver disease. The impact of HCV infection in all-cause mortality has been explored, showing an odds ratio for liver and cardiovascular mortality of 11.6 (CI 95%: 5.54–24.4), p<0.0001, and 2.15 (CI 95% from 1.58 to 2.91), p<0.001, respectively.55
The survival of the HCV+ patient with combined liver -kidney transplant (CLKT) is greater than that observed in sequential transplants “Liver Transplant (LT) after RT” or “RT after LT”.56 In patients with HCV and HIV coinfection, the HCV infection is the major determinant of patient survival, suggesting that in these patients HCV treatment is a priority.57
Graft survivalSeveral studies, mostly retrospective, have shown that after RT patients HCV+ show significantly lower survival than HCV−.1,2,17,48–52 Spanish studies of HCV infection in RT patients using the database of “Chronic Graft Nephropathy” show that the 10 years graft survival censoring for death was 69% in HCV+ vs 79% in HCV−.46 A metanalysis showed that the presence of antibodies anti-HCV is an independent risk factor for graft survival44 in 4 of the 8 studies included in the analysis (relative risk 1.56 [CI 95%: 1.35–1.80], p=0.019).52 This finding has been confirmed in a recent systematic review based on 18 observational studies.53
Proteinuria due to interstitial fibrosis and tubular atrophy (IFTA), HCV-associated glomerulonephritis, TGN and NDRT may contribute to the decrease in graft survival. Accordingly, HCV viremia has been associated with greater frequency of IFTA and reduced graft survival in patients with HCV RNA+ at the time of RT.58,59
Graft survival in combined Liver and renal transplant is superior than the obtained with RT after LT or LT after RT.56 In RT patients co-infected with HCV-HIV, graft survival is lower than in RT patients with HIV+.57
Management and treatment of HCV in dialysis or renal transplantation based on interferonTherapeutic management of HCV in dialysis “The era of interferon”Until 2014, before the availability of the new direct-acting antiviral drugs (DAA), the HCV treatment in patients with ESRD was based on INF±Rib60 (Table 1).
Treatments of HCV approved in Spain.
| Until August 2014: |
| - Dual therapy interferon plus ribavirin for genotypes 1, 2, 3, 4, 5 and 6. |
| - Triple therapy (pegylated interferon, ribavirin and a HCV protease inhibitor, a direct action antiviral of the first generation, boceprevir (Victrelis®) or Telaprevir (INCIVO®) only for HCV genotype 1 |
| Since August 1, 2014 for genotype 1 and 4: |
| - Triple therapy with simeprevir (Olysio®) plus pegylated interferon and ribavirin. |
| - In November 2014 the Spanish Agency of Medications (AEMPS) published the position report for therapeutic use (IPT) of simeprevir |
| - In November 2014, AEMPS published the IPT of sofosbuvir (Sovaldi®) |
| - On February 20, 2015, AEMPS published the IPT of daclatasvir (Daklinza®) |
| On March 20, 2015, AEMPS published the IPT on: |
| - Ledipasvir/sofosbuvir (Harvoni®) |
| - Viekirax® (ombitasvir/paritaprevir/ritonavir) and Exviera® (dasabuvir) |
| On February 3, 2017, AEMPS published the IPT on: |
| Elbasvir and grazoprevir (Zepatier®) |
| On June 8, 2017, AEMPS published the IPT of: |
| - Sofosbuvir and velpatasvir (Epclusa®) |
| On March 12, 2018, AEMPS published the IPT of: |
| - Glecaprevir and pibrentasvir (Marivet®) |
| Approved by the FDA in July 2017, pending approval in Spain: |
| - Sofosbuvir/velpatasvir/voxilaprevi r (Vosevi®) |
As said, HCV infection in RT is associated with worse patient and graft survival as compared to patients without infection.24 Given the risk of INF to induce renal graft dysfunction,61,62 international guidelines had recommended treating HCV infection before RT. This was the treatment before direct-acting antiviral drugs became available.1,63
Despite the recommendations to treat HCV infection before RT, only 1% of patients HCV RNA+ hemodialysis received treatment with IFN as reported in the study DOPPS. This is, a very low percentage probably due to the poor tolerance and poor efficacy of this antiviral regime.64
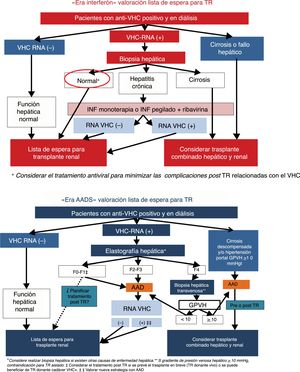
International guidelines have recommended that patients with active HCV infection should be evaluated for the degree of liver fibrosis, and until a few years ago only liver biopsy was available for such evaluation. Likewise in patients with cirrhosis, it is necessary to determine the presence of portal hypertension; if a gradient of hepatic venous pressure is ≥10mmHg and/or esophageal varices are observed, the consensus of guidelines have considered that isolated RT should not be performed and the possibility of combined Liver and renal transplant should be considered65 (Fig. 1).
Antiviral treatment of dialysis patients with HCV infection in the interferon era included INF-pegylated for 48 weeks for genotypes 1, 4 and for 24 weeks for genotypes 2 and 3. During the treatment, patients were excluded from the waiting list, and once virus was removed after completion of therapy it was recommended to wait at least 28 days before receiving a RT.1,66
A meta-analysis of 287 patients treated with INF and Rib showed a sustained viral response (SVR) in 60% of the patients with 18% abandonment due to side effects, especially anemia and infections.67 With these results it was assumed that in the pre-DAA era, the INF-pegylated and Rib were the standard treatment of HCV in dialysis patients.
Patients who were unable to clear up the RNA virus with antiviral treatment could be included in the waiting list for TR, given the fact that the life expectancy of patients with HCV replication is higher if they are transplanted than if they remain on dialysis.41–44,68
Post-transplant renal antiviral treatment “interferon era”A meta-analysis of 16 clinical studies showed that the sustained viral response was 34% and the abandonment of treatment was 32%.67
The most frequent and serious side effect of treatment with INF in RT patients was graft dysfunction, usually as steroid-resistant acute rejection, which required discontinuation of the treatment.1,56,69
During the INF era KDIGO guidelines advised treatment with IFN only in cases of RT in which the benefits of antiviral therapy were greater than the risks, such as cholestatic fibrosing hepatitis, rapidly evolving cirrhosis or de novo or recurrent cryoglobulinemic MPGN.1
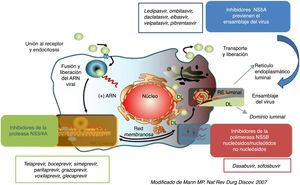
Direct-acting antivirals in the treatment of HCVTherapeutic strategies against HCV using DAAThe great advance in the knowledge of the HCV life cycle and the characteristics of the proteins of this virus has facilitated the development of new DAA that act specifically on the HCV life cycle70 (Fig. 2).
According to the phase of the life cycle in which the DAA acts to prevent HCV replication, they DAA are classified as protease inhibitors (PI), inhibitors of NS5A replication complex and NS5Bpolymerase inhibitors.71
Since 2014, new DAA have been commercialized60 (Table 1), achieving a SVR greater than 95% in the general population.
The first DAAs used in the treatment of HCV were the IP NS3/4A telaprevir72 and boceprevir,73 that were approved for the treatment of HCV 1 genotype in 2011 in combination with INF-pegylated and Rib.8 Although the SVR rate obtained was a 75% of cases, the cost, side effects and complexity of its administration was limitation in the use of these antivirals.
The launch of the first generation of IP NS3/4A served as a springboard for the development of new DAAs, such as NS5B polymerase inhibitors, inhibitors of NS5A viral replication complex and second generation PIs with a great improvement in efficacy and security.7,8,74
In United States, year 2013 and in Europe, February 2014, were approved three new DAA simeprevir (IP-NS3/NS4A), daclatasvir (Inhibitor-NS5A) and sofosbuvir (polymerase-NS5B inhibitor). Having these new DAAs available allowed the development of different therapeutic strategies against HCV that were safe and efficient and without the need of INF. This has been a great advance in the treatment of HCV, and especially for the patients such as RT in which the use of INF is contraindicated.
This initial antiviral therapeutic scheme against HCV entails the combination of two or three of these new drugs given orally, with or without Rib, but without INF, during a period of 12–24 weeks depending on factors such as the virus genotype, the degree of hepatic fibrosis, the previous history of anti-HCV treatment and renal function. These therapies have achieved a SVR of 90–100%, with a good safety profile.75,76
The next stage in the development of new strategies against HCV started in 2015 with the use of combinations of DAA after the approval of new formulations without INF or Rib60 (Table 1). The fixed combination of sofosbuvir (SOF) with ledipasvir (LDV) was approved by the FDA in October 2014 and in Spain in March 2015. In the last 3 years there has been a continuous incorporation of new DAAs, such as elbasvir (EBR) glecaprevir (GLE), asunaprevir, paritaprevir (PTV), grazoprevir (GRA), pibrentasvir (PIB), velpatasvir (VEL), ritonavir (r), ombistavir (OBV), dasabuvir (DSV) and voxilaprevir (VOX)60,75 (Table 1).
The fast research progress in this field has allowed to outspread the therapeutic coverage to all HCV genotypes (pangenotype), reducing the duration of treatment, improving the safety profile and minimizing pharmacological interactions77 (Table 2).
Recommended combinations for treatment against HCV (general population).
| HCV combinations | Antiviral mechanism | Drug excretion | Genotype efficacy (GT) | Duration of treatment (weeks) | Kidney failureeGF<30ml/min/1.73m2 | Most common side effects | Posology |
|---|---|---|---|---|---|---|---|
| SOF/VEL | |||||||
| SOF sofosbuvir | NS5B inhibitor | SOF→renal | → 1a, 1b, 2, 4, 5, 6 | 12 | SOF not recommended | Headache, fatigue and nausea | 400mg/100mg one tablet per day |
| VEL velpatasvir | NS5A inhibitor | VEL→biliary | GT3 if absence of cirrhosis | ||||
| SOF/VEL/VO X | |||||||
| SOF sofosbuvir | NS5B inhibitor | SOF→renal | → 1a, 1b, 2, 3, 4, 5, 6 | 12 | SOF not recommended | Headache, fatigue and nausea | 400mg/100mg/10mg one tablet per day |
| VEL velpatasvir | NS5A inhibitor | VEL→biliary | for GT3 with cirrhosis | ||||
| VOX voxilaprevir | NS3-4A inhibitor | VOX: Diarrhea | |||||
| SOF/LDV | → 1b. Naïve and not naïve | ||||||
| SOF sofosbuvir | NS5B inhibitor | SOF→renal | → 1a, 4, 5, 6. Only naïve | 8–12 | SOF not recommended | Headache, fatigue and nausea | 40mg/90mg one tablet per day |
| LDV ledipasvir | NS5A inhibitor | LDV→biliary | |||||
| OBV/PTVr+DSV | OBV/PTVr | ||||||
| OBV ombitasvir | NS5A inhibitor | OBV→fecal | [→ 1b. Naïve and not naïve (with or without cirrhosis) | 8–12 | Do not need to adjust dose in renal failure | Fatigue and nausea: watch interaction ritonavir with immunosuppresive agents | 50mg/75mg/12.5mg one tablet per day |
| PTV partaprevir | NS3-4A inhibitor | PTV→fecal | + | ||||
| Rit ritonavir | CYP3A inhibitor | DSV→liver metabolism | DSV 250mg two times at day | ||||
| DSV dasabuvir | Inhibit NS5B | ||||||
| GZR/EBR | |||||||
| GZR grazoprevir | NS3-4A inhibitor | Biliary and fecal excretion | → 1A naïve and no naïve | 8–12 | Do not adjust dose in renal failure | Headache, fatigue, elevation transaminases | 100mg/50mg one tablet per day |
| EBR elbasvir | NS5A inhibitor | Yes | |||||
| RNA <800,000IU/ml | |||||||
| → 1b independent of RNA | |||||||
| → 4 only naive with RNA <800,000IU/ml | |||||||
| GLE/GDP | |||||||
| GLE glecaprevir | NS3-4ª inhibitor | Biliary excretion | → 1a, 1 b, 2, 3, 4, 5, 6 | 8–12 | Do not adjust dose in renal failure | Headache, fatigue | 100mg/40mg one tablet per day |
| GDP pibrentrasvir | NS5A inhibitor | ||||||
Naïve: no previous antiviral. Not naïve: pretreated with interferon, pegilated interferon, ribavirina+sofosbuvir.
In general, the tolerance to the new DAAs is acceptable and with only a few side effects (Table 2). Antivirals, alone or in combination, such as SOF, SOF/LDV, SOF/VEL, GRA/LDV and GLE/PIB, are associated with fatigue and headache.76 Headache, diarrhea and nausea have been the most frequent side effects with SOF/VEL/VOX, these gastrointestinal manifestations are superior to those observed with the double combination SOF/VEL.78
Using the new DAA, without INF, the cure of HCV is achievable in all genotypes, regardless of the presence of cirrhosis. However, one of the main problems is the risk of interactions with other drugs, which may decreased efficacy or increased toxicity.
The most relevant interactions are through the cytochrome P450 enzyme (CYP450). Interactions with glycoprotein P (P-gp) and with breast cancer resistance protein (BCRP) also have the potential to affect the bioavailability of the drug.79,80 Ritonavir, a potent CYP3A4 inhibitor, if co-administered with drugs metabolized by this enzyme may result in a marked increase in plasma concentrations. Therefore, before planning the antiviral treatment, it is advisable to evaluate the specific data sheet of the drug to have a detailed description of the interactions with other medications.
The use of some inducers of CYP450 and P-gp is contraindicated in all regimes because of the risk of significantly decreasing DAA concentrations. On the other hand, regimes that include an NS3-4A IP, such as PTV-r, GRA, GLE or VOX, should not be used in patients with decompensated cirrhosis Child-Pig B or C, because the increased risk of high IP concentration with the consequent toxicity.78
In relation with renal function, there are only recommendations for the use of NS5B SOF polymerase inhibitor, since its elimination is mainly through the kidneys, so it is not recommended if the estimated glomerular filtration rate (eGFR) is <30ml/min/1.73m2, unless there are no other therapeutic options, and if used it should be under close monitoring.78
Immunosuppressive drug interactionsOne main concern with the use of some antiviral drugs in RT is the potential interaction of some DAAs with immunosuppressive drugs which may result in rejection of the transplanted organ is (Table 3).
Adjustments of immunosuppressant dose in patients treated with DAA.
| Antiviral | Azathioprine | Mycophenolate | Cyclosporine | Tacrolimus | Sirolimus | Everolimus |
|---|---|---|---|---|---|---|
| Sofosbuvir (Sovaldi®)Simeprevir (Olysio®)Daclatasvir (Daklinza®)Sofosbuvir/ledipasvir (Harvoni®)Paritaprevir/ombitasvir/ritonavir (Viekirax®) and Dasabuvir (Exviera ®)Sofosbuvir/velpatasvir (Epclupsa®)Sofosbuvir/velpatasvir/voxilaprevir (Vosevi®)Grazoprevir/elbasvir (Zepatier®)Glecaprevir/Pibrentasvir (Marivet®) | ––––––––– | –––––Monitor–––– | –Do not prescribe–Monitor Monitor–Do not prescribeDo not prescribeDo not prescribe | –Monitor–Monitor Monitor–MonitorMonitorMonitor | –Monitor–MonitorMonitor–MonitorMonitorMonitor | –Monitor–MonitorMonitor–MonitorMonitorMonitor |
−: no clinical interaction.
Monitor: potential interaction that requires dose adjustment or additional monitoring.
Cyclosporine is an inhibitor of OATP1B1/3, BCRP and P-gp, as well as inhibitor of cytochrome CYP3A, so the co-administration with IP NSA3-4A alters the elimination of the antiviral causing an increase in plasma concentration. Thus, the concomitant use of both drugs is not recommended, while co-administration of simeprevir-tacrolimus produces small increases in the exposure of the NS3-4A and a small decrease in the levels of tacrolimus making advisable a close monitorization of the plasma levels of the inhibitor of calcineurin.81–85
With the new combinations containing an IP NS3-4A, GRA/EBR, GLE/PIB, SOF/LDV/VOX, there may be changes in exposure and plasma levels of tacrolimus and, although dose adjustment is not required it is necessary a close plasma monitoring of the immunosuppressant (see specific data sheet).86–89 The interaction of these drugs with imTOR has not been well studied, so it is necessary to monitor their plasma levels until more information is available.
Daclatasvir, SOF and the fixed combination SOF/LDV or SOF/VEL can be safely administered with calcineurin inhibitors tacrolimus/everolimus and imTOR sirolimus/everolimus.90
With regard to the combination of OMB/PTVr/DSV with the different immunosuppressive drugs, the strong inhibition of CYP3A4 and P-gp produced by ritonavir is the main cause of high exposure to calcineurin inhibitors and/or imTOR, being necessary to monitor the corresponding immunosuppressant.81 One study reported that the dose of cyclosporine was reduced to a fifth of the basal dose and tacrolimus was changed from 0.5mg/weekly to 0.2mg/48h to maintain levels within the therapeutic range.91
It has been described that after removal of the HCV in transplant patients there is a fall in the plasma levels of the calcineurin inhibitor,92,93 which has been related to the improvement of liver function and the consequent increase in the metabolism of the inhibitor of calcineurin.94 According to Sawinsky et al.,92 45% of patients required an increase in the dose of calcineurin inhibitors during antiviral treatment, maintaining this dose after the end of treatment.
In summary, the availability of new DAAs in INF-free guidelines offers the possibility of effectively treating HCV+ RT patients. In spite of the adequate safety profile of the DAAs, the potential interaction of these drugs with some immunosuppressants, as well as the improvement of liver functionalism after eradicating HCV, demands a close plasma monitoring of the immunosuppressant to minimize the risk of rejection, so it is essential to have a collaboration of hepathologist and nephrologist in the follow-up of patients.
Direct-acting antivirals and dialysisAccording to the RUBY-1 study the combination OMB/PTVr/DSV for the treatment of genotype1 in patients with advanced CKD without cirrhosis produced a SVR of 90%.95
The clinical trial phase III, C-Surfer, included 122 patients with CKD stage 4 and 5 (6% cirrhotic and 20% not naïve) with genotype 1HCV treated with oral combination GRA/EBR for 12 weeks. The response achieved excluding 6 patients with non-viral failure was 99.1%, and 94.2% in the complete analysis96 (Table 4).
Most relevant clinical trials based on interferon-free therapeutic schemes for the treatment of HCV in dialysis or kidney transplant patients.
| Study | Status failure renal | With or without cirrhosisGenotype (GT) | Therapy | Duration (weeks) | Sustained viral response-SVR 12 |
|---|---|---|---|---|---|
| C-SURFER (Roth et al.) | FG<30ml/min (75% dialysis) | With and without Cirrhosis.GT 1, naïve and no naïve | GRA/EBR | 12 weeks | 99% (94%) |
| RUBY-1 (n=20)(Pockros et al.) | CKD stage 4 (GFR 15–30ml/min/1.73m2)Stage 5 CKD (GFR <15ml/min/1.73m2 or on dialysis) | GT1Without cirrhosis | PTVr-OBV-DSV | 12 weeks | 90% |
| Colombo et al.Phase II (n=114) | RT with GFR>40ml/ml/min/1.73m2 | With or without cirrhosisNaïve and not naïveGT1GT4 | LDV/SOF | 12–24 weeks | 100% |
| EXPEDITION-4 (n=104) (Gane et al.) | CKD stage 4–5 | GT1-6 naïve and no naïveGT1 (52%)GT2 (16%)GT3 (11%)GT4 (19%)GT5 or 6 (2%) | GLE/GDP | 12 weeks | 98.1% |
| MAGELLAN-2 (n=100) (Reau et al.) | RT (n=20) and Liver Transplant (n=80) | GT 1–6 naïve and no naïveGT3 only naiveWithout cirrhosis | GLE/GDP | 12 weeks | 98% |
Naïve: no previous antiviral treatment; No naïve pretreated with interferon, pegylated interferon, ribavirin+sofosbuvir.
DSV: dasabuvir; ELB: elbasvir; GRA: grazoprevir; OBV: ombitasvir; PTVr: paritaprevir-ritonavir.
The GLE/GDP combination was evaluated in the Expedition-IV trial, a 12week treatment of patients with genotypes 1 to 6, naïve or with a previous treatment with INF, and in patients with CKD stages 4 or 5. This combination obtained an SVR of 98%97 (Table 4).
Based on these results, these combinations have been regularly recommended to treat HCV in patients with advanced CKD and on maintenance dialysis.78,81
Direct-acting antivirals and renal transplantPioneering studies have shown excellent results in the treatment of HCV in RT patients with SOF regimens with,92,93,98,99 achieving a SVR of 100% after 12 weeks.
Based on data from the Spanish renal transplant registry, in 103 patients treated with DAAs in different regimens without INF, the SVR12 was 98%.98 The most commonly used combinations were SOF/LDV (57%) and SOF/daclatasvir (17%), and 41% of patients received Rib. Immunosuppressive adjustment was required in 55% of patients. No significant changes were detected in proteinuria and renal function before and after treatment; although 3 patients had acute humoral rejection likely in the context of a reduced exposure to immunosuppressive treatment.98
A multicenter randomized phase II including 114 TR patients with HCV genotype with 1 and 4 with the combination SOF/LDV for 12 or 24 weeks achieved a SVR of 98%86; 18% of patients required adjustment in the immunosuppressive dose without episodes of rejection. Accordingly, in patients with good renal function, the SOF-based regimen has been efficient and safe (Table 4).
To choose the right combination of DAA in RT patients, it is necessary to take into account the renal function, and the HCV genotype77,78 (Tables 5 and 6).
Recommended combinations for HCV treatment in kidney disease.
| Kidney disease stage | GT1a | GT1b | GT2 | GT3 | GT4 | GT5 | GT6 |
|---|---|---|---|---|---|---|---|
| → GLE/GDP | → GLE/GDP | → GLE/GDP | → GLE/GDP | → GLE/GDP | → GLE/GDP | → GLE/GDP | |
| If estimated GFR <30/ml/min/1.73m 2 and in patients on dialysis | 8–12 weeks→ GZR/EBR12 weeks YesRNA 800,000UI/ml (5.9Log 10/ml) | 8–12 weeks→ GZR/EBR12 weeks→ OBV/PTVr + DSV12 weeks | 8–12 weeks | 8–12 weeks | 8–12 weeks→ GZR/EBR12 weeks) if RNA≤800,000UI/ml (5.9Log10/ml) | 8–12 weeks | 8–12 weeks |
| → SOF/LDV | → SOF/LDV | → SOF/VEL | → SOF/VEL | → SOF/LDV | → SOF/LDV | → SOF/LDV | |
| Renal transplant with estimated glomerular filtration >30/ml/min/1.73m2Renal transplantWith estimated glomerular filtration <30/ml/min/1.73m2 | 8–12 weeks→ SOF/VEL12 weeks→ GLE/GDP12 weeks | 8–12 weeks→ SOF/VEL12 weeks→ GLE/GDP12 weeks | 12 weeks→ GLE/GDP8–12 weeks | 12 weeks→ GLE/GDP12 weeks | 8–12 weeks→ SOF/VEL12 weeks→ GLE/GDP12 weeks | 8–12 weeks→ SOF/VEL12 weeks–→ GLE/GDP12 weeks | 8–12 weeks→ SOF/VEL12 weeks→ GLE/GDP12 weeks |
Naïve: no previous antiviral treatment; No naive: pretreated with interferon, pegylated interferon, ribavirin+SOF.
DSV: dasabuvir; EBR: elbasvir; GLE: glecaprevir; GT: genotype; GZR: grazoprevir; LDV: ledipasvir; OBV: ombitasvir; GDP: pibrentasvir; PTV: paritaprevir; r: ritonavir; SOF: sofosbuvir; VEL: velpatasvir; HCV: hepatitis C virus; VOX: voxilaprevir.
Recommendations for the treatment of HCV infection in renal transplant patients.
| • All kidney transplant patients with active HCV infection should be evaluated to receive antiviral treatment |
| Regime with DAA |
| Treatment with interferon should not be considered |
| The choice of treatment should be based on the HCV genotype, viral load, interaction with other drugs, GFR, degree of liver fibrosis and comorbidities. |
| • Before treatment, an evaluation should be performed to assess the interaction of DAA drugs with the concomitant medication, including the immunosuppressant drugs |
| • Use protocols that have shown efficacy and safety of the regimes based on DAA in the renal transplant patients. |
| • The following should be monitored during and after the treatment with DAA. |
| Calcineurin inhibitors |
| Renal function |
| Proteinuria |
The phase II study, MAGELLAN-II included 100 patients with genotype 1, 2, 3, 4, 5 or 6 without cirrhosis with liver transplant (80%) or renal transplant (20%) treated for 12 weeks with GLE/GDP. Except for the genotype 3 (only naïve), naïve and non-naïve (prior treatment with regimens including INF, peg-INF, RBV±SOF) were included, achieving an SVR at 12 weeks in 98% of patients (Table 4).
Recommendations for the treatment of HCV in patients with RT are detailed in Table 6. During and after completion of DAA treatment patients should be monitored for calcineurin inhibitor levels,77,92,93 renal function88 and proteinuria. It should be noted that glomerular segmental and focal hyalinosis has been reported after treatment with DAA.89
Management of patients on the waiting list for kidney transplant with HCV infection, “the AAD era”The recommended treatment before or after RT will depend on the type of donor, the time on waiting list for a given type of donor, the possibility of using organs from HCV+ donor, the HCV genotype and the degree of liver fibrosis77 (Fig. 1).
As previously mentioned, all HCV patients who are candidates for RT cirrhosis should be evaluated using liver biopsy or a non-invasive method to stratify the degree of liver fibrosis.77,78 In patients with compensated cirrhosis without portal hypertension, isolated RT may be considered, while combined liver -renal transplant is recommended in decompensated cirrhosis and/or severe portal hypertension.
It is important to consider what is the optimal time to perform the antiviral treatment while on the waiting list for transplantation. Except in cases of decompensated cirrhosis or significant portal hypertension, if the RT is expected to be performed soon (within 6 months approximately) from living donor the AAD treatment may be programmed post-RT. Conversely, if the RT is planned beyond 24 weeks, it must be assessed the risk-benefit of deferring treatment after transplantation.77
Likewise, in patients on the waiting list for RT from a cadaveric donor, antiviral treatment may be deferred after RT to allow for the possibility of receiving a donor kidney HCV+ and reduce waiting time,66 especially if the immunological risk is low and the possibility of receiving soon a compatible donor is high. However, patients without the possibility of being transplanted as an HCV+ donor, or in those with low chances of being offered a compatible graft soon, antiviral treatment should be offered before the RT.
It is important to consider that in patients who remain HCV+ while on the waiting list, an annual evaluation of the degree of liver fibrosis should be performed, and if there is evidence of progression of liver damage, antiviral treatment should be initiated.
The type of genotype may also influence the decision on what is the optimal time to perform antiviral treatment. Until now, in patients on the waiting list for RT infected by genotype 1 or 4, antiviral treatment is indicated before RT using GRA/EBR or OMB/PTVr/DSV in the case genotype 1b. However for genotype no-1 or no-4 the treatment is postponed after the TR, since the regimes approved to date have been based on SOF. With the recent FDA approval of the GLE/PIB pangenotype combination, without the need for adjustment in renal insufficiency, treatment can be planned in all cases before RT78 (Table 5).
Patients on the waiting list with cirrhosis despite becoming HCV- should be assessed every 6 months with ultrasound to rule out the occurrence of hepatocellular carcinoma.96
HCV and HBV co-infectionIn HCV and HBV co-infection, the serum level of HBV-DNA is often undetectable, with HCV being the main causative agent of hepatic inflammatory activity, so with the clearance of HCV there is a risk of HBV reactivation.
It is essential to determine the serological status for HBV before starting treatment against HCV and in patients with HCV-HBV coinfection, concomitantly with anti-HCV therapy, it should be started treatment against HBV with nucleoside/nucleotide analogs.78 If the HBsAg if positive, specific treatment against HBV should be performed for 12 weeks after completing treatment with AAD. If HBsAg is negative but anti-core HBV is positive, the HBV-DNA and HBsAg should be monitored in case of increasing or not normalizing transaminases (monthly determinations) during or after HCV treatment, and initiate anti-HBV therapy if HBsAg and/or HBV-DNA are detected.78
Renal transplantation using kidneys from donors with HCVRenal transplantation of kidneys from donor HCV+ in receptor HCV+After demonstrating that organ transplantation can transmit HCV,100 there was general consensus that the kidneys of HCV+ donors should not be transplanted in HCV− patients.101 However, due to organ shortage, it was raised the possibility of transplanting kidneys from HCV+ donors into HCV+ recipients.102,103
The first experiences in RT using HCV+ donor kidneys in HCV+ receptors, in which our country was a pioneer, demonstrated that in short term this strategy was feasible and safe.103–106 As a consequence, national and international guidelines recommended this practice to avoid loss of organs.
Despite these recommendations, RT using HCV+ donor kidneys in HCV+ receptors has continued but remains controversial.104–107 American studies, mostly based on analysis of registries, show a shorter waiting time for RT but it was associated with a small increase in the risk of death, graft loss and severe liver disease, compared to those receiving kidneys from HCV− donors.108–111 These authors suggest that the high incidence of new onset diabetes after transplant (NODAT) in HCV+ receptors may explain the decrease in patient survival. However, the survival of patients receiving kidneys from HCV+ donors is higher than HCV+ patients maintained on the waiting list.77,104–107
The cooperative study between the hospitals October 12 of Madrid and Clínic of Barcelona, in RT patients from HCV+ donors in HCV RNA+ receptors, reported that at a long term a positive donor serology is not an independent risk factor for liver disease or grafts and patient survival.21 These results have been reproduced in recent studies, also pointing out that the shorter waiting list time contributes to improved graft survival (censored death) compared to HCV+ recipients transplanted with kidneys from HCV−112 donors. Therefore, the KDIGO guidelines continue to recommend transplantation with HCV+ donors into HCV+ RNA receptors.77
In recent years there has been a decrease in the incidence and prevalence of HCV infection on dialysis, so that the offer of a donor kidney HCV+ may sometimes not be used due to lack of an adequate recipient,110 suggesting that organizational measures should be taken, including the offer of these kidneys for early RT.109
As recently suggested, the success of DAAs therapy will help to increase the use of the HCV+ donor kidneys (Fig. 1). It is being proposed to transplant HCV+ patients with mild fibrosis with an HCV+ donor kidney and treat them after RT with the new DAA,4 so that the period of time waiting for transplant would be shortened. In addition, there are already experiences of HCV+ receptors of a HCV+ kidney that were treated with DAA and achieved the eradication of the virus after transplantation.113
In short, the use of HCV+ donor kidneys in HCV+ RNA receptors is a long-term safe strategy that provides a patient survival which is longer than that of HCV+ patients who remain on the waiting list for RT. The availability of DAAs therapy after TR, will help to increase the pool of donors by using the HCV kidneys in HCV+ receptors throughout the world. However, antiviral treatment in advanced CKD or on dialysis patients, in countries with universal or almost universal access to antiviral treatment will reduce or may even make to disappear the number of possible HCV+ receptors; actually this is happening in Spain (data not published).
Renal transplantation with kidneys of HCV+ donors in HCV recipients: is it feasible in the era of new antivirals?The waiting list for RT is increasing every year in most countries, and the waiting time for transplantation is too long. In this context, the question that has emerged in the transplant community is: “In the new era of antivirals, RT with HCV+ donor kidneys in HCV receptors; is it feasible and ethically acceptable?”.113,114
Two North American trials using kidneys from HCV+ donors in HCV receptors− have been the pioneers in assessing the possibility and safety of this therapeutic option.115,116 The THINKER trial included 10 patients HCV− receiving a kidney donor HCV RNA+ with genotipe 1. Antiviral treatment with grazoprevir/elbasvir was administered when viremia was evidenced which occurred around the third day posttransplant. All patients became infected, and after 12weeks of treatment all showed SVR.115 Likewise the EXPANDER trial included 8 patients on, grazoprevir/elbasvir, preoperatively and after transplant, and again all patients became infected and they all had SVR after 12 weeks of treatment.116 The data published by Goldberg et al.,115 is being completed and expanded with the recent work by Reese et al.,117 showing the results of 20 HCV− patients after receiving a HCV RNA+ kidney transplant obtaining a 100% eradication of the virus at 12 month treatment. A recent study also confers economic advantages in favor of using HCV RNA+ kidneys for RT to HCV− receptors compared to HCV− patients on regular dialysis on the waiting list for a HCV− donor.118
The strategy of using HCV RNA+ donors in HCV receptors− offers the possibility of increasing the pool of donors for RT and thus reducing the waiting list119; however, although the protocols have been already established in some national and international centers, the experience is still scarce.
Although these experiences are promising, caution should be maintained until more complete information is available. In fact, the HCV Consensus Conference of the American Transplant Society recommends that the transplantation of HCV+ donor organs into HCV recipients− should be performed only as a research protocol with a thorough and rigorous informed consent that must be authorized by the ethical committee of each center.101
Financial supportThe present work has not received any specific financial support from public agencies or commercial or nonprofit sectors.
Conflicts of interestThere are no conflicts of interest.
Please cite this article as: Esforzado N, Morales JM. Hepatitis C y trasplante renal: el tiempo de la erradicación del virus ha llegado. Nefrología. 2019;39:458–472.