Antecedentes: Pocos trabajos han estudiado la asociación entre el genotipo de la haptoglobina (Hp) y el riesgo de nefropatía diabética (ND) en pacientes con diabetes tipo 1 (DM1), con resultados contradictorios hasta ahora. Objetivos: Estudiar si el genotipo 2-2 de Hp se asocia a un incremento del riesgo de ND en población española con DM1. Métodos: Se diseñó un estudio de casos y controles. CASOS: pacientes con DM1 y enfermedad renal crónica estadio 5 de la NKF-KDOQI, en espera de trasplante reno-pancreático o que han sido trasplantados (reno-pancreático o renal aislado). CONTROLES: pacientes con DM1, apareados por sexo y tiempo de evolución de la diabetes, con función renal y excreción urinaria de albúmina normales. El genotipo de Hp se realizó mediante reacción en cadena de la polimerasa y electroforesis. Resultados: Incluimos 57 casos y 57 controles, sin diferencias estadísticamente significativas en el sexo (70 % frente a 61 % varones, p = 1,0) o duración de la diabetes (23,0 ± 6,7 frente a 20,8 ± 9,3 años; p = 0,1), aunque la edad de inicio de la diabetes fue menor en los casos (14,1 ± 6,8 frente a 17,7 ± 10,1 años, p = 0,03). La frecuencia de genotipos 1-1, 1-2 y 2-2 fue de 19,3 %, 42,1 % y 38,6 % en los casos y de 17,5 %, 49,1 % y 33,4 % en los controles, respectivamente, sin diferencias significativas (p = 0,8). El análisis de regresión logística condicional no mostró asociación entre el genotipo 2-2 de Hp y el desarrollo de ND (OR 1,14, IC 0,52-2,52). Conclusiones: En nuestra muestra de población española con DM1, no se ha hallado asociación entre el genotipo de Hp y el riesgo de ND.
Background: Few reports have studied the possible association between the haptoglobin (Hp) genotype and the risk of diabetic nephropathy (DN) in type 1 diabetes (T1D), with conflicting results to date. Aims: To study whether the 2-2 Hp genotype is associated with an increased risk of overt DN in a Spanish population with T1D. Methods: We performed a case-control study in a Spanish population. CASES: T1D patients with end-stage renal disease (stage 5 of NKF-KDOQI), awaiting reno-pancreatic transplantation or having already been transplanted (reno-pancreatic or renal alone). CONTROLS: T1D patients, matched for sex and time of diabetes evolution, with preserved renal function and normal urinary albumin excretion. Hp genotyping was done using polymerase chain reaction and electrophoresis. Results: We included 57 cases and 57 controls in the study. There were no statistically significant differences in gender (70% vs. 61% males, p=1.0) or the duration of diabetes (23.0±6.7 vs. 20.8±9.3 years; p=0.1), although the age of onset of diabetes was lower in the cases (14.1±6.8 vs. 17.7±10.1 years, p=0.03). The frequency of genotypes 1-1, 1-2 and 2-2 was 19.3%, 42.1% and 38.6% in cases and 17.5%, 49.1% and 33.4% in controls, respectively, with no statistically significant differences between groups (p=0.8). Conditional logistic regression analysis showed no significant association between genotype 2-2 of Hp and the development of DN (OR 1.14, CI 0.52-2.52). Conclusions: In our sample of a Spanish population with T1D, no association was found between the Hp genotype and risk of overt DN.
INTRODUCTION
Haptoglobin (Hp) is a protein that acts as an antioxidant due to its ability to combine with haemoglobin and prevent tissue damage caused by free haemoglobin. The Hp gene is polymorphic, with two types of alleles, named 1 and 2, resulting in three potential genotypes of Hp: Hp 1-1, Hp 2-1 and Hp 2-2.1 The protein derived from allele 2 of Hp provides less antioxidant activity than the protein of allele 1.1,2
There is increasing evidence in medical literature of the association between the genotype of Hp and cardiovascular disease (CVD).3 In this respect, various longitudinal epidemiological studies have found a risk of CVD 2 to 8 times greater in patients with diabetes mellitus with the Hp genotype 2-2, both in type 1 diabetes mellitus (T1D) as well as in type 2 (T2D).4-7
The association between the Hp genotype and microvascular complications in diabetes has been less studied. Until now, few studies have focused on the implications of Hp in the risk of diabetic nephropathy (DN), using heterogeneous populations of patients with T1D, with contradictory results to date.8-11
In light of these findings, the objective of our study is to investigate whether the genotype 2-2 of Hp is associated with an increased risk of DN in a Spanish population with T1D.
MATERIAL AND METHOD
A case-control study was designed for a Spanish population. All the patients were selected from the Biobank database (DNA bank) from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and from the Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders (CIBERDEM), a technical and scientific infrastructure that coordinates the obtaining, processing, storage and provision of biological samples (http://www.clinicbiobanc.org/en_index.html). The cases were defined as all the patients registered in the Biobank with T1D and stage 5 chronic renal failure of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative, awaiting reno-pancreatic transplantation or having already been transplanted (reno-pancreatic or renal alone).
The controls were defined as patients with T1D and preserved renal function (estimated glomerular filtration [eGFR] > 90ml/min/1.73m2) and normal urinary excretion of albumin, paired with the cases by sex and time of diabetes evolution (matching by frequency). T1D evolution time was defined as the years between the diagnosis of diabetes and the date on reaching an eGFR < 15ml/min/1.73m2 (for the cases) and between the diagnosis of the illness and the entry date into the database of Biobank (for the controls).
The clinical data collected were: sex, age, age at diagnosis of T1D and duration of T1D until entry into the Biobank database (controls) or until the transplant or renal replacement therapy (cases). Hp genotyping was carried out using samples of DNA supplied by Biobank via polymerase chain reaction following the protocol of Koch et al.12.
The study protocol was performed in agreement with the Declaration of Helsinki and was approved by the Ethics Committee of both Hospital Clínic i Universitari de Barcelona and Biobank. Informed consent of the subjects of the study was obtained.
Statistical analysis was carried out using the STATA.11 statistical package. Numerical variables are shown as means and standard deviations for the continuous variables, and as a number and percentage for categorical variables. The Student’s t-test was used for the comparison of continuous variables and the chi-squared test for categorical variables. Logistic regression models were constructed in order to study the association between the genotype 2-2 of Hp and DN. The Hardy-Weinberg equilibrium study for the genotype of Hp was analysed with 1 degree of freedom. Statistical significance was established at P values <.05.
RESULTS
114 patients were included in the study, 57 cases and 57 controls. There were no statistically significant differences in sex (70 % vs. 61 % males, P=1.0) or the duration of diabetes (23.0 ± 6.7 vs. 20.8 ± 9.3 years, P=.1). However, the cases were older (44.9 ± 8.6 vs. 40.9 ± 9.5 years, P=.02) and the age at onset of diabetes was lower (14.1 ± 6.8 vs. 17.7 ± 10.1 years, P=.03).
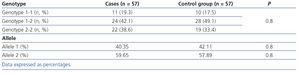
The distribution of genotypes in our sample was in Hardy-Weinberg equilibrium. The prevalence of the genotype 1-1, 2-1 and 2-2 of Hp was 19.3 %, 42.1 % and 38.6 % in cases, and 17.5 %, 49.1 % and 33.4 % in controls, respectively. There were no differences in the frequency of the different genotypes or in the frequency of the different alleles between cases and controls (Table 1). The logistic regression study, including sex and the time of T1D evolution as covariables in the model, did not show a statistically significant association between the genotype 2-2 of Hp and the development of DN (odds ratio 1.14, confidence of interval 0.52-2.52).
DISCUSSION
In this case-control study in a Spanish population, we did not find a association between the Hp genotype and the presence of DN in patients with T1D, despite careful patient selection, representing the extremes of the DN spectrum with the same time of diabetes evolution.
The contribution of the genotype of Hp to the risk of CVD has been analysed in various longitudinal studies, relating the genotype 2-2 with an increased risk of CVD in patients with T1D and T2D.4-7 This association could have some implications in the treatment of patients with diabetes, given that vitamin E supplement has shown to reduce cardiovascular events,13,14 mostly in those patients with T2D.
With respect to microvascular complications of diabetes, this association is less consistent. In fact, there have been few studies on the relation between the genotype of Hp and the risk of diabetic retinopathy, with contradictory results to date15,16.
Some studies have studied the association between the Hp genotype and the risk of DN, but only four included patients with T1D.8-11 The first study,8 which included patients both with T1D and T2D, showed a significant increase of the genotype 2-2 in patients with micro- and macroalbuminuria versus the other genotypes. The second study (case-control)9 included 509 Irish patients with T1D, defining DN as the presence of proteinuria above 0.5g/24h. This study did not show statistically significant differences between the three genotypes, although a higher frequency of allele 2 of Hp was discovered in the cases. The third study10 included 95 patients with T1D and 170 with T2D, without finding an association between the genotype of Hp and the presence of DN (defined as the presence of micro- or macroalbuminuria). The last,11 and the only prospective study, included 486 patients with T1D, with a mean follow-up of 18 years. No relation was found between the genotype of Hp and the risk of DN in univariate models, although in multivariate models a risk nearly two times greater of a decrease in eGFR and of chronic kidney disease with the genotype 2-2 of Hp was discovered.
In light of the different definitions used for DN in different studies, we decided to carry out a genetic study in patients with no indication of DN (controls) and to compare them with those with a more serious form of DN (cases), with the same duration of T1D. The frequency of the different genotypes of Hp was very similar to that of previous studies which have analysed the frequency of genotypes of Hp in a Spanish population17. However, we have not found a link between the genotype 2-2 and a higher frequency of DN (38.6 % in cases and 33.4 % in controls; P=.8) or a protective effect of the genotype 1-1 (19.3 % in cases and 17.5 % in controls; P=.8).
Our study is not without its limitations. Given that our cases had very restricted selection criteria, our sample of patients with T1D is limited. In addition, we do not know the longitudinal information about the metabolic control or other confounding factors that could influence the evolution of DN, such as smoking or hypertension.
In conclusion, in our sample of Spanish patients with T1D, the genotype of Hp did not appear to have an implication for the risk of DN. In light of our results and the contradictory results from other studies, more studies are needed, with a wider sample group and of prospective nature, in order to clarify the role of Hp in the risk of microvascular complications in T1D.
ACKNOWLEDGEMENTS
We would like to thank Biobank of IDIBAPS and CIBERDEM for the samples and for the technical assistance provided.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Haptoglobin genotype and frequencies of the alleles in the population of the study