Antecedentes: No hay suficiente evidencia sobre la frecuencia de rechazo agudo y la función del injerto en los pacientes con retiro temprano de esteroides (RTE). El objetivo del presente estudio es comparar el efecto del RTE sobre la tasa de filtrado glomerular (TFG), la supervivencia/rechazo del injerto en receptores de una cohorte de tratados con tacrolimus/mofetil micofenolato, comparada con un grupo control. Material y métodos: Cohorte retrospectiva en 60 receptores de bajo riesgo inmunológico entre diciembre de 2005 y julio de 2010. Cohorte del estudio (C-RTE; N = 32), el RTE se hizo el 5º día mientras recibían tacrolimus/mofetil micofenolato. La cohorte control (C-C, N = 28) recibió prednisona/tacrolimus/mofetil micofenolato. Las variables clínicas, bioquímicas e histológicas fueron evaluadas al inicio del estudio, y a los 3, 6 y 12 meses de seguimiento. Se utilizó Kaplan-Meier y el modelo de riesgos proporcionales de Cox para evaluar la supervivencia. Las comparaciones entre cohortes fueron hechas por la t de Student y c2. Resultados: Durante el seguimiento, la C-C muestra presión sanguínea significativamente mayor tanto sistólica (125 ± 10 frente a 114 ± 8) como diastólica (81 ± 8 frente a 72 ± 7), glucosa sérica (96 ± 13 frente a 86 ± 10), triglicéridos (177 ± 61 frente a 129 ± 34), colesterol total (183 ± 43 frente a 148 ± 34) y colesterol LDL (100 ± 22 frente a 87 ± 25). La C-C presentó una mayor proporción de uso de antihipertensivos (57 frente a 13 %) y de estatinas (27 frente a 9 %). La TFGe fue mejor en la C-RTE que en la C-C (85,4 ± 20,6 frente a 70,6 ± 17,0, p = 0,004). La frecuencia de rechazo agudo fue menor en la C-RTE. Conclusiones: La supervivencia del injerto, la TFG, la tasa de rechazo agudo y el perfil metabólico fueron mejores en la C-RTE que en la C-C.
Background: Acute rejection and graft function have not been completely clarified in early-steroid-withdrawal (ESW) patients. The objective of this study was to compare the effect of early steroid withdrawal on GFR, graft survival/rejection in recipients in a cohort treated with tacrolimus/mycophenolate mofetil compared to a control cohort. Material and method: Retrospective cohort, in 60 low immunological risk recipients between December 2005 and July 2010. Study cohort (ESW-C N=32), steroid withdrawal was carried out after 5 days, while they were receiving tacrolimus/mycophenolate mofetil. The control cohort (C-C, N=28) received prednisone/tacrolimus/mycophenolate mofetil. Clinical, biochemical and histological variables were assessed at baseline and after 3, 6, and 12 months of follow-up. Kaplan-Meier and the Cox proportional hazards model were used to assess survival. Comparisons between cohorts were carried out by the Student’s t and c2 tests. Results: At follow-up, C-C displayed significantly higher systolic (125±10 vs. 114±8) and diastolic (81±8 vs. 72±7) blood pressure, serum glucose (96±13 vs. 86±10), triglycerides (177±61 vs. 129±34), total (183±43 vs. 148±34) and LDL-cholesterol (100±22 vs. 87±25). C-C had a higher proportion of antihypertensive (57 vs. 13%), and statins (27 vs. 9%) use. eGFR was better in ESW-C than in C-C (85.4±20.6 vs. 70.6±17.0, p=.004). AR frequency was lower in ESW-C. Conclusions: Graft survival, GFR, AR rate and metabolic profile were better in the ESW-C than in C-C.
INTRODUCTION
One of the great challenges in kidney transplant management is acute rejection (AR) but with the new immunosuppression regimen, the frequency of AR has decreased in recent decades, and we have observed a significant long-term improvement in graft and patient survival.1,2 In Mexico, graft and patient survival is comparable to that reported in other countries.3,4 However, there is still a considerable loss of renal grafts due to patient death with a functional graft (up to 40%),5 in which cardiovascular disease is the main cause of mortality. Unfortunately, corticosteroids (as the basis for post-transplant immunosuppression) have been directly associated as one of the major cardiovascular risk factors.6,7
The prevalence of diabetes mellitus, obesity and metabolic syndrome in the general population is increasing,8,9 and the risk of developing them after transplantation with the use of corticosteroids could be higher, thus leading to an increase in morbidity and mortality. In fact, the use of these drugs is associated with de novo diabetes after renal transplantation.10 A strategy aimed at reducing these comorbidities is avoiding the use of, or the withdrawal of corticosteroids (whether it be late or early), after transplantation, whenever the risk of AR and graft survival are not compromised; however, the results in this regard have been controversial.11-13
Our population and probably some others are at greater risk of developing diabetes mellitus and other complications after renal transplantation, and as such, strategies for avoiding or reducing risk factors in this type of patient are necessary. Therefore, the objective of this study was to compare the effect of early steroid withdrawal (ESW) on the estimated glomerular filtration rate (eGFR), graft survival, frequency of AR and the metabolic profile in a renal transplantation cohort (ESW-C) treated with tacrolimus (TAC) and mycophenolate mofetil (MMF), in comparison with a control group (C-C) treated with the internationally recommended triple immunosuppression regimen (TAC, MMF and PDN).
MATERIAL AND METHOD
A retrospective cohort study was carried out on 32 renal graft recipients at the CMNO, IMSS Specialist Hospital in whom corticosteroid treatment was withdrawn. These patients received transplantations between December 2005 and July 2009, and were followed up until July 2010. During the study period, a total of 989 renal transplantations were performed (48 deceased donor), mostly from related living donors. Most patients selected received transplants from living donors (58/60) and all had a low immunological risk (panel reactive antibody against HLA classes I and II <30%). The ESW is an intervention that is very infrequently performed in our hospital and the decision to withdraw corticosteroids was taken by the nephrologist in accordance with the patient’s characteristics and their experience. We included all of the patients for whom corticosteroids were withdrawn during this period (ESW-C); the C-C consisted of recipients who had the same transplantation time, immunosuppression regime and immunological risk. Patients with a history of using corticosteroids before transplantation, diabetes mellitus, delayed renal graft function (those requiring renal replacement therapy immediately after transplantation) and multiple organ transplantation were excluded.
All patients received our hospital’s standard induction immunosuppression regimen based on: 0.18mg/kg/day TAC (divided into two doses), 2g/day MMF (divided into two doses), 500mg intravenous methylprednisolone (MPD) (peri-operatively) and 20mg of basiliximab peri-operatively and four days after transplantation. Oral maintenance therapy was performed with 2g/day MMF and 0.15mg/kg/day TAC, with an adjustment in accordance with serum levels (9-15ng/ml in the first 30 days and 8-10ng/ml during follow-up).
The ESW was carried out as follows: one day before and during transplantation (peri-operatively), patients received 500mg/day MPD, followed by 250, 125, 60 and 30g/day in days 1, 2, 3 and 4, respectively. The total withdrawal of steroids was carried out five days after transplantation. The C-C received maintenance immunosuppression with prednisone, in accordance with international recommendations, combined with TAC and MMF.
The following clinical and biochemical variables were obtained from medical registries in the post-transplantation phase: age, sex, family history of diabetes mellitus, personal history of pregnancy (females), cause of kidney disease, type of and time on renal replacement therapy, weight, height, blood pressure, glucose, total cholesterol, total cholesterol linked to high-density lipoproteins (HDL) and cholesterol linked to low-density lipoproteins (LDL), triglycerides, HLA, risk of infection by the cytomegalovirus (CMV), time of ischaemia and use of antihypertensive drugs and/or statins.
All assessments were carried out after 3, 6 and 12 months, and subsequently, each year in the post-transplantation period, and information was collected, such as: time of transplantation, immunosuppressant maintenance dose, episodes of AR, type and dose of anti-rejection therapy (MPD or polyclonal antibodies), medication (antihypertensive drugs, statins, hypoglycaemic agents). Risk of CMV infection was classified in accordance with the serological result of the ELISA for CMV (IgG): high risk: positive donor/negative recipient; intermediate risk: positive donor/positive recipient; low risk: negative donor/negative recipient.14,15
eGFR was calculated using the formula MDRD-416 (MDRD [ml/min/1.73m2] = 186x [creatinine/88.4]-1,154 x age-0,203 x 0.742 if the patient was female). The results of protocol biopsies were recorded (which have been carried out for ten years in our hospital as a matter of course, 3, 6 and 12 months after transplantation); furthermore, we recorded indicated biopsy results (whether due to renal dysfunction or proteinuria) and all the biopsies were assessed by the same nephrologist using the Banff histopathological classification.17 The study was assessed and approved by the Local Research and Ethics Committee.
Statistical analysis
Data are displayed as mean ± standard deviation or numbers and percentages. The Student’s t-test and χ2 tests were used for comparisons between groups. Graft survival was assessed by Kaplan-Meier and the comparisons were carried out using the Log-rank test. The Cox proportional hazards model was used to assess the influence of steroid withdrawal at the start of AR. The statistical analyses were carried out using the SPSSä version 17 software (SPSS, Inc., Chicago, IL).
RESULTS
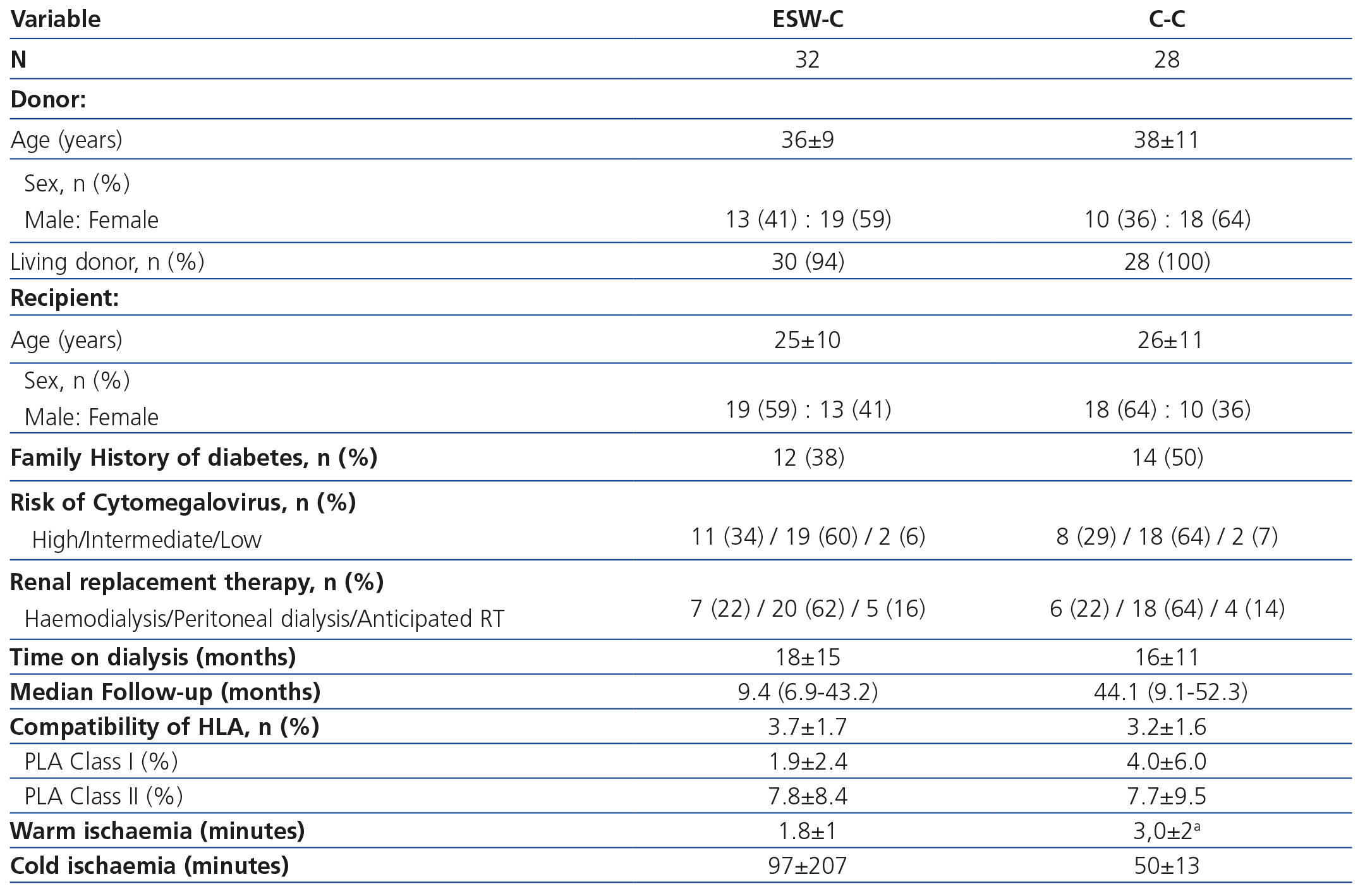
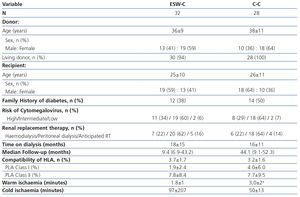
The transplantation demographic characteristics are displayed in Table 1. We did not find differences between the groups in terms of the age or gender of the donor and recipient, the type of donor, a history of diabetes, the risk of CMV, the date and type of dialysis or compatibility of the HLA. Two grafts were transplanted from deceased donors in the ESW-C and the rest of the grafts came from living donors. Warm ischaemia was shorter in the ESW-C, while the C-C had a longer follow-up period.
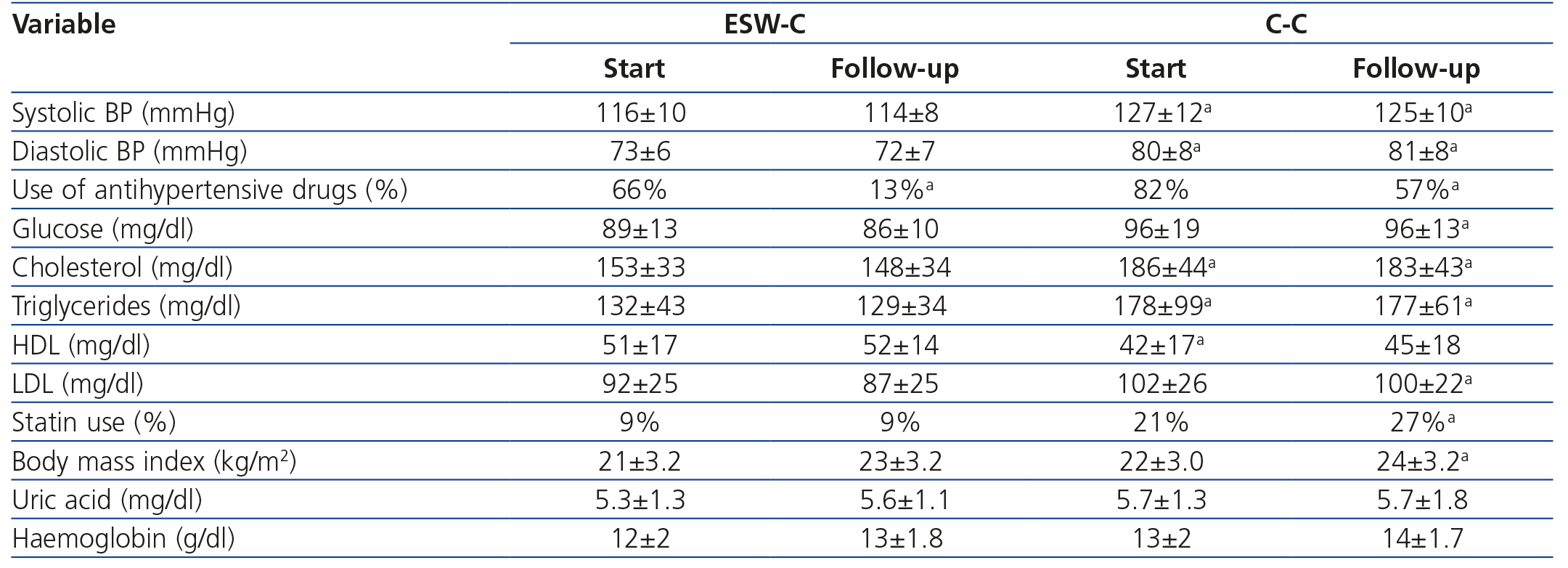
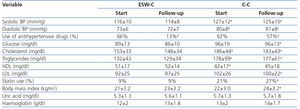
The clinical and biochemical comparisons at the start of the study and at follow-up are displayed in Table 2. At the start of the study, ESW-C patients had significantly lower blood pressure, lower total cholesterol and triglycerides and higher HDL concentrations than the controls, although, the percentage of patients who used antihypertensive drugs and statins was not significantly different. At the end of follow-up, patients in the C-C had higher blood pressure, glucose, triglycerides, total cholesterol and LDL and a higher percentage of statin use than those of the ESW-C. At the end of follow-up, only one patient developed PTDM and this patient was in the C-C.
Curiously, the percentage of patients who used antihypertensive drugs decreased significantly in the ESW-C from 66% to 13% at the end of the study, which was significantly lower than the percentage of use in the C-C.
Graft function and acute rejection
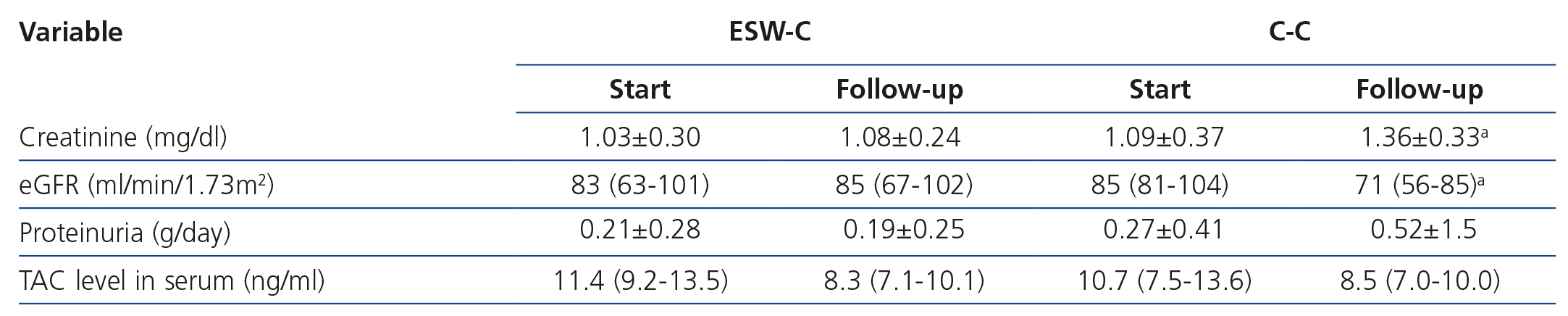
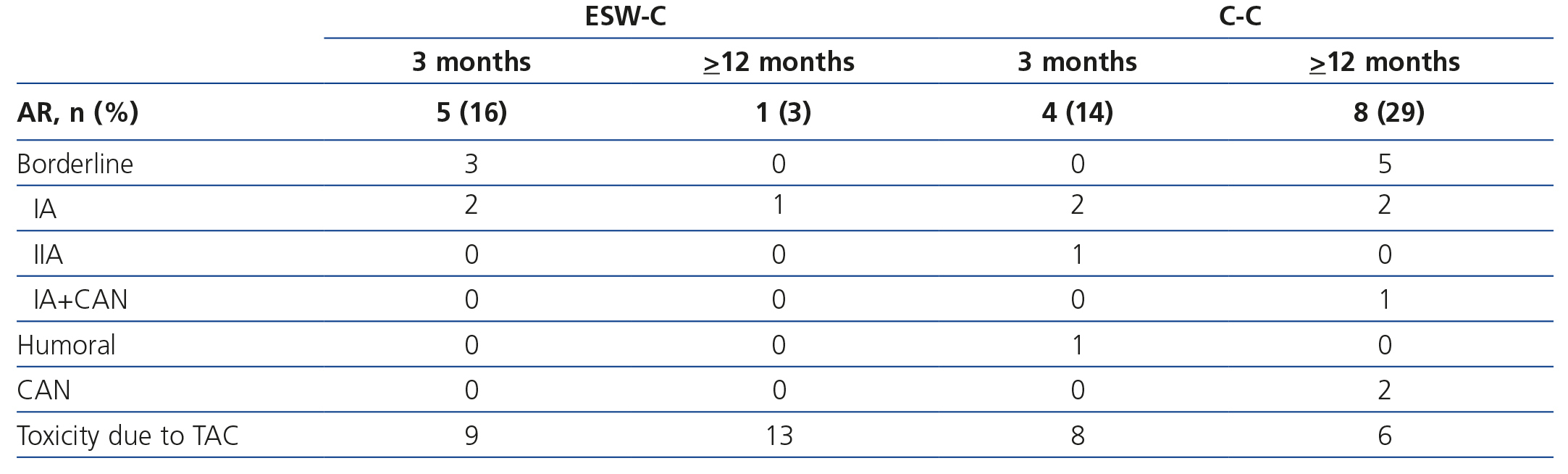
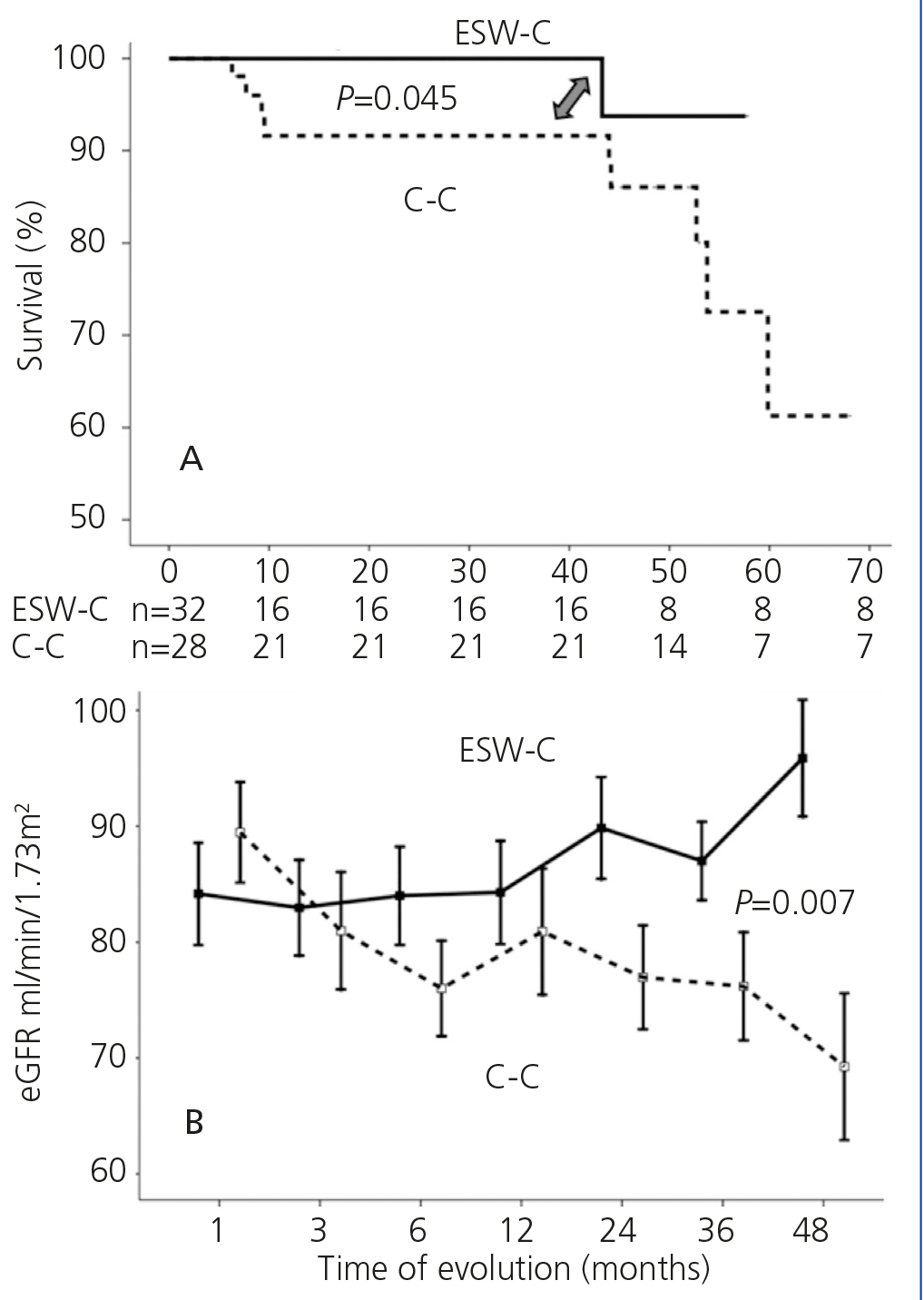
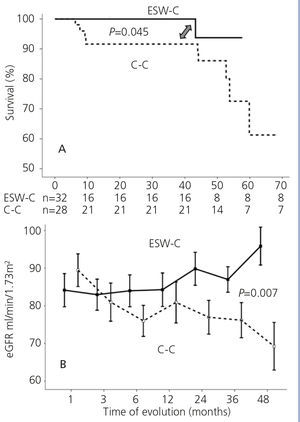
Graft survival (defined as a loss of ≥30% of GFR) is displayed in Figure 1A. ESW-C patients had significantly better renal function than the controls (p=.04). When we compared renal function (measured by serum creatinine or eGFR), there were no differences between the cohorts in the baseline assessment; however, at the end of follow-up, ESW-C patients had better graft function (Figure 1B and Table 3). With regard to the histopathological results (# biopsies indicated for each cohort), there were 9 cases (15%) of AR in the first 3 months and 9 cases (30%) at the end of follow-up (Table 4). The accumulated incidence of AR was significantly lower (P=.04) in the ESW-C (19%) than in the C-C (43%). The Cox proportional hazards model was used to assess potential predictors of AR, such as independent variables included in the immunosuppression regimen (ESW 1, control 0), HLA, age, sex and serum cholesterol. However, none of these variables were significant independent predictors of AR (c2 model 2.63, P=.76).
Table 4 displays the histopathological results of the renal biopsies. Nine episodes of AR were found in the protocol biopsies carried out within the first 3 months after transplantation; of the patients in the ESW-C, 3 had borderline AR and 2 had Banff IA AR, while the controls had 2 Banff IA AR, 1 Banff IIA AR and 1 acute humoral rejection. In the biopsies carried out after between 9 and 12 months (whether by protocol or indicated), of the patients in the ESW-C, 1 had Banff IA AR, while of those in the C-C, 5 had borderline AR and 3 had Banff IA AR. The latter findings were significantly different between cohorts (P=.006). Three C-C patients had chronic vascular disease of the graft in the biopsies conducted after 1 year of follow-up. Moreover, toxicity due to TAC was found in 23 patients (36 events of toxicity): 14 patients (61%) of the ESW-C and 9 patients (39%) of the C-C (P=.21). Of these, 10 patients (4 of the ESW-C/6 of the C-C) were changed to sirolimus and the GFR was not different between these two groups (72.31±22.80, 74.37±19.75, p=.82), and none had AR.
Despite the higher frequency of toxicity by TAC (confirmed by biopsy) in the ESW-C, levels of the drug in blood were not significantly different between the cohorts and they were within the therapeutic range in all cases (Table 3).
DISCUSSION
Corticosteroids are currently used in most renal transplantation centres around the world. However, there is a growing tendency to avoid their use due to their side effects and the negative impact on graft and patient survival, even at low doses.18-20 Results of various studies in which steroids have been withdrawn have been controversial, probably due to the heterogeneity of the different immunosuppression protocol used, based on cyclosporine, TAC, azathioprine, MMF, rapamycin or everolimus,12,21 with or without antibodies (monoclonal or polyclonal).13,22-24 In the Latin American population, there are no data available for this intervention, especially with homogeneous immunosuppression regimens based on TAC and MMF. Nowadays, there is a documented higher risk in the development of obesity and diabetes in various populations,8,9 which could be exacerbated after renal transplantation (and corticosteroids could be a major risk factor). In our setting, a higher risk of de novo diabetes after transplantation was associated with corticosteroid use;10 medical interventions such as ESW may be beneficial in transplant recipients. Obviously, this intervention must not increase the risk of AR or decrease renal function. Initially, only patients with a low immunological risk and who ideally use potent immunosuppressive drugs would be candidates for steroid withdrawal.
We included patients who met these requirements and our results were similar to those of other studies carried out in similar conditions.25 One of the main concerns with the withdrawal of corticosteroids was the greater risk of AR. The results of some studies that have reported a high rate13 of AR are questionable, since a significant number of them did not perform renal biopsies (gold standard) or even reported the methodology for diagnosing AR, which could explain the results of a higher AR rate. In this study, AR was documented with protocol or indicated biopsies, and as such, both subclinical and clinical rejections were identified. Our results with ESW did not predict the development of AR or the incidence of AR during the first year and this was consistent with that reported by other hospitals.26 Most late episodes of AR were documented in biopsies carried out due to an increase in serum creatinine and were more common in C-C (29%) than in ESW-C (3%). A possible explanation for this result is that the ESW-C had higher toxicity due to TAC documented histopathologically than the C-C, in spite of patients in the ESW-C receiving lower doses of TAC (data not displayed). From a pharmacological point of view, corticosteroids are cytochrome P450 inducer stimulants (isoenzyme CYA4) responsible for the biotransformation of TAC, and in ESW, this favours greater bioavailability of TAC, which may explain why patients with ESW had greater toxicity due to TAC and a lower rate of AR.27,28 In the C-C, the eGFR was lower in comparison with that of the ESW-C, which could be explained by the higher rate of AR observed in this group during follow-up. The withdrawal of steroids could potentially improve the haemodynamic and metabolic profile. González-Molina et al.29 retrospectively assessed 923 kidney recipients (deceased donor) with steroid withdrawal and found lower blood pressure, total cholesterol and triglycerides. In this study, both groups had an appropriate control of blood pressure and serum lipids; however, ESW-C achieved this control with the use of a lower dose and frequency of drugs and these results were consistent with other studies.13 These results have encouraged us to continue this intervention in our population through clinical trials to test these findings.
CONCLUSION
In patients with a low immunological risk, with an immunosuppression regimen based on TAC and MMF, ESW does not change graft survival, renal function or the rate of AR in this cohort. Toxicity due to TAC (confirmed by biopsy) tends to be more common in ESW-C than in the controls and the reason for this merits further research. ESW-C patients had a lower requirement for antihypertensive drugs and hypolipidaemic agents in comparison with the C-C, which suggests a better metabolic profile. Our findings require further clinical trials in homogenous populations in order to avoid controversy with this type of intervention.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Demographic and transplantation characteristics.
Table 2. Clinical and biochemical comparisons between the cohorts at the start and end of follow-up.
Table 3. Comparison of renal function between cohorts at the start and end of follow-up.
Table 4. Histopathological findings in renal biopsies carried out after 3 or &
Figure 1. Comparison of survival in accordance with the study cohort. B. Comparison of renal function in each cohort.