To the Editor,
Multiple myeloma (MM) is a clonal proliferation of plasma cells with the production of monoclonal immunoglobulins. This disease can be diagnosed as a result of a variety of clinical manifestations, including bone pains (lytic lesions), a major increase in plasma proteins, and/or the presence of monoclonal protein in blood/urine samples, and signs and symptoms indicative of malignancy, including anaemia, hyperviscosity syndrome, hypercalcaemia, and renal failure. Previous studies have mentioned mortality rates between 10% and 20% in the first two months of its appearance.1
Here we describe the case of one patient, previously healthy, with recently diagnosed oligosecretory MM, who developed fulminant symptoms one week after detection, having just received the first dose of chemotherapy.
The patient was a 69-year old female. The only relevant history was a peptic ulcer several years before, and the patient did not take any medications chronically or have a family background of kidney disease. The patient was also waiting for an operation to treat a crural hernia. Two months before seeking emergency treatment, the patient’s plasma creatinine level was 0.9mg/dl, with no anaemia, and with normal urine parameters and chest x-ray results.
The patient was admitted to the emergency room with a compromised general state of health, anorexia, and nausea.
Anamnesis did not indicate a decreased water intake, but the rhythm of diuresis did, as well as the appearance of nocturia, which previously had not occurred. The patient also had a cough with bloody sputum, dyspnoea upon light exertion, and orthopnoea.
Upon questioning, the patient commented that she had received an anti-flu shot one month prior, and had later started treatment with oral calcium and paracetamol (this treatment had been abandoned in the last 15 days).
The physical examination indicated general poor state of health. The patient had a baseline O2 saturation of 78% and jugular ingurgitation. Pulmonary auscultation revealed crepitation up to mid-level, and the abdomen was globular with pitting oedema in the limbs. The rest of the physical examination was normal.
The blood analyses carried out in the emergency room revealed: creatinine: 5.5mg/dl, Na: 136mEq/l, K: 5.4mEq/l, calcium: 10.9mg/dl, pH: 7.30, HCO3: 19mEq/l, haemoglobin: 8.8g/dl, haematocrit: 27%, leukocytes: 13 300, and platelets: 133 000. The coagulation analysis was normal.
Urine analysis (systematic) resulted in: proteins ++, blood +++, sediments >40 red blood cells/field, ionogram: Na 41mmol/l, K 52mmol/l.
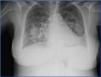
A chest x-ray taken when the patient was hospitalised indicated bilateral increased densities with butterfly patterns (Figure 1), and the abdominal ultrasound detected homogeneous hepatosplenomegaly and kidneys with normal size and morphology, with no dilation of the urinary tract.
With the available data indicating rapidly progressing renal failure, anaemia, and bloody sputum, as well as the increased densities in the chest x-ray, we suspected extracapillary glomerulonephritis and started treatment with 500mg pulses of 6-methylprednisolone. We also started diuretic treatment with a positive response, but with no improvement in renal function, and so we also started renal replacement therapy with haemodialysis.
The laboratory blood analysis (ordinary) indicated that the patient had urea concentrations of 185mg/dl, uric acid: 13mg/dl, cholesterol: 254mg/dl, triglycerides: 218mg/dl, GOT: 44U/l, GPT: 81U/l, GGT: 222U/l, albumin: 4.5g/dl, total protein: 6.6g/dl, calcium: 10.4mg/dl, phosphorous: 7.6mg/dl, and ferritin: 915ng/ml. Viral serology for hepatitis B (HBV), hepatitis C (HCV) and human immunodeficiency virus (HIV) was negative. The immunological analysis demonstrated a normal complement with a decrease in immunoglobulins: immunoglobulin IgG: 174mg/dl (normal: 751-1560), IgA: 8mg/dl (normal: 82-453), and IgM: 8mg/dl (normal: 46-304).
Aiming to clarify the cause of the renal failure, we performed a renal biopsy and found: seven glomeruli per plane (one sclerosed); the other glomeruli had a mesangial expansion matrix with occasional accumulations resembling nodular lesions. Immunofluorescence was negative for immunoglobulins IgG, A, M, C3, and kappa, and positive for lambda in the intratubular sample. We also observed acute interstitial tubular necrosis in the regeneration phase and, most notably, acellular eosinophilic content in the tubules with an accompanying giant cell reaction. There was no interstitial fibrosis or vascular abnormalities, and the final diagnosis was compatible with a myeloma kidney.
After receiving the results of the biopsy, the immunological analysis resulted normal (ANA, ANCA, anti-MBG). In the electrophoretic spectrum we detected two monoclonal peaks with a slight monoclonal component (0.2g/dl). Proteinuria in urine samples at 24 hours was 0.13g/24 hours, and the electrophoresis of the urine sample detected the elimination of monoclonal light chains (kappa elimination: free kappa: 5.6mg/dl).
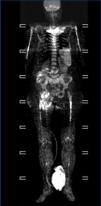
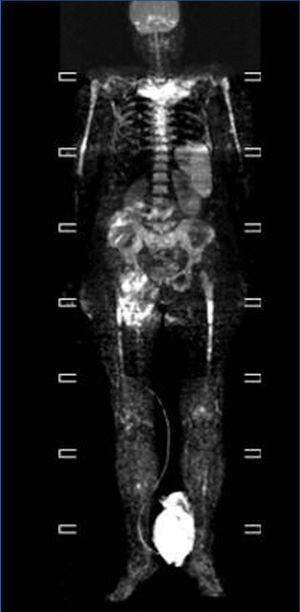
We consulted with the haematology department and performed a bone marrow biopsy that was compatible with a type MM malignant monoclonal gammopathy with intense damage and probable compacted bone marrow. The metastatic bone analysis only showed osteoporosis, and did not detect lytic lesions. The full-body MRI (Figure 2) demonstrated extensive infiltration into the marrow including in the cranium, spinal column, and diaphysis of the long bones.
We also performed a cardiological evaluation including the following components: electrocardiogram (upon hospitalisation) revealing a sinusal rhythm of 100bpm, negative T in DI, aVL, and V6. Echocardiogram: ejection fraction (EF) at 60%, mild concentric hypertrophy in the left ventricle (LV) and mild mitral failure.
With the diagnosis of IgG kappa multiple myeloma with urine elimination of free kappa in the oligosecretory range, we decided on a treatment regimen including bortezomib at 1.3mg/m2 intravenously on days 1, 4, 8, and 11, and dexamethasone at 40mg/day orally on days 1-4 and 9-12.
Two days after receiving the first dose of chemotherapy, and coinciding with a potassium level of 7.5mEq/l, the patient suffered severe hypotension, with an escape rhythm of 20bpm. We administered emergency dialysis and inserted a pacemaker, in spite of which we continued to detect heart rhythm abnormalities, requiring a permanent pacemaker. The patient continued with progressive deterioration and severe hypotension, requiring the administration of noradrenaline and mechanical ventilation, finally resulting in patient death two weeks after hospitalisation.
Renal involvement in MM is common, and there is a correlation between the presence and severity of renal failure and patient survival.2 Cast nephropathy (myeloma kidney) is the most common development in patients with MM and renal dysfunction.3 Protein M is not detected in approximately 3% of patients with MM in the immunofixation analysis of blood and urine samples upon diagnosis, considering these cases to be non-secretory MM; it is rare that these patients should have cast nephropathy, since there is very little light chain elimination.4
In our case, this being an oligosecretory MM (with little monoclonal presence in blood and urine samples), the renal histological analysis detected the presence of cast nephropathy (myeloma kidney), which led to the diagnosis of MM. The bone marrow biopsy confirmed MM with widespread involvement. The bone analysis also did not detect images indicative of osteolysis, although the full-body MRI did find diffuse myeloma involvement (Figure 2).
It is also curious that in spite of the low detection of tumour mass and the previous good health of the patient (with no prior cardiological incidents), cardiac arrhythmias started to appear (initially related to toxic hyperkalaemia possibly associated with tumour lysis due to the chemotherapy); however, after halting chemotherapy and intensifying the haemodialysis treatment and implanting the pacemaker (temporary and permanent), the cardiac arrhythmias persisted and the patient died.
Our objective here was to communicate the case of discordance between an oligosecretory MM with a minimal monoclonal component and very extensive spread that led to patient death one week after diagnosis.
Conflicts of interest
The authors have no conflicts of interest to declare.
Figure 1. Chest x-ray obtained upon patient hospitalisation
Figure 2. Full-body magnetic resonance. Figure 1. Evolution of calcium, phosphorous and intact parathyroid hormone (iPTH) levels.