To the Editor,
The increase of adynamic bone disease in haemodialysis (HD) patients can hinder the diagnosis of other diseases that progress with latent hypercalcaemia. Here we present a n elusive relevant case.
A male patient of 73 years with terminal renal failure (TRF) secondary to diabetic nephropathy that started HD in June 2008. The patient smoked 10 cigarettes/day, and did not consume alcohol. The patient had long-term arterial hypertension (AHT), type 2 diabetes mellitus for 45 years on treatment with insulin, diabetic retinopathy, dyslipidaemia, chronic ischaemic heart disease tending towards acute myocardial infarction (AMI), stage III b-IV chronic ischaemia of the lower limbs requiring femoropopliteal bypass in December 2009, 80% stenosis of the left carotid artery, widespread vascular calcifications, chronic bronchopathy, obstructive sleep-apnoea syndrome, small (lacunar infarcts of the thalamus and hemiprotuberance) and large (previous stroke in the left hemisphere) vessel ischaemic brain disease, and polyarthrosis with severe degeneration of the lumbar spinal column.
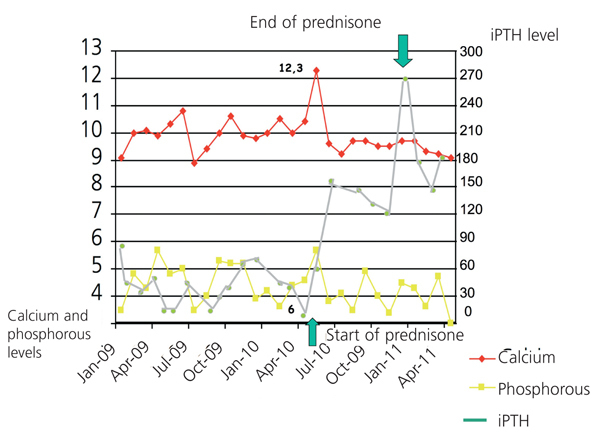
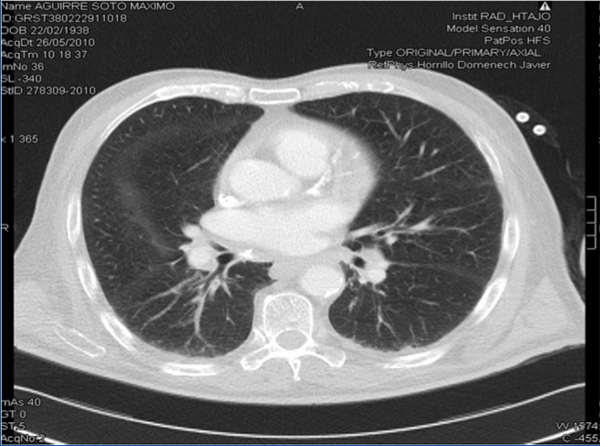
Three years before starting HD, and with treatment including thiazide and oral calcitriol, the patient was hospitalised for severe hypercalcaemia (13.5mg/dl), with neurological and digestive symptoms. In the subsequent analysis, we observed hilar lymph nodes of a non-pathological size and isolated reticular pulmonary parenchymal opacities, homogeneous hepatosplenomegaly, angiotensin-converting enzyme (ACE) on the upper limit of its normal range (three measurements taken between 30U/l and 60U/l, under normal conditions, normal range: 8-55). All other tests were negative, and the patient was diagnosed with exogenous intoxication by vitamin D and thiazide, and was treated with parenteral pamidronate, with an excellent clinical evolution and no levels of 1,25 (OH)2 vitamin D. Since then, the patient has been asymptomatic, with suppressed intact parathyroid hormone (iPTH) levels and a spontaneous tendency towards hypercalcaemia, for which he was diagnosed with adynamic bone disease (ABD). During the month of July 2009, the patient suffered a progressive condition of asthenia, anorexia, night sweating, disorientation, irritability, amnesic alterations, apathy, apraxia, and motor difficulty for walking. We also observed cortico-subcortical atrophy, leukoaraiosis, and dilation of the ventricular system, and after performing a lumbar puncture with negative biochemical and microbiological results, we ruled out normotensive hydrocephalus, infectious disease, and degeneration of the central nervous system (CNS). The patient’s symptoms were explained by small-vessel encephalopathy and a conservative treatment plan, evolving slowly towards chronicity. We did not administer oral calcium, vitamin D, or its derivatives, with dialysis provided with a calcium bath at 2.5mEq/l. In May 2010 the patient had no clinical variations, with hypercalcaemia at maximum values of 12.2mg/dl and iPTH at 6pg/ml (Figure 1). We performed a complementary study with the following results: normal thyroid profile, negative Mantoux (negative Booster), chest x-ray indicative of chronic obstructive pulmonary disease (COPD), normal levels of parathyroid hormone-related peptide (PTHrP), normal alkaline phosphatase levels, levels of 1,25 (OH)2 vitamin D reduced to 10pg/ml, normal plasma protein immunoelectrophoresis and proteinogram, negative immunology, bone scan and imaging demonstrated osteopaenia and vascular calcifications with no indications of osteolysis, normal tumour markers, and ACE at 157U/l. The full-body computed tomography (CT) showed several bilateral mediastinal hilar lymph nodes (Figure 2). Pulmonary fields had ground glass densities with nodular morphology in the middle lobe, periportal, perigastric lymph nodes and in the celiac trunk and left para-aortic and interaortocaval spaces, and spirometry with mixed ventilation abnormalities that were mostly restrictive in nature.
We detected bronchitis and chondritis in the bronchoscopy. We then performed a bronchoalveolar lavage and mediastinal fine-needle aspiration with negative cytology for malignancy and no granulomas or lymphoid cellularity. The culture for mycobacteria also resulted negative. The lymphocytic sub-populations in the outflow from the lavage were within normal ranges, with a CD4/CD8 index of 2.22.
Diagnosed with pulmonary sarcoidosis, the patient was treated with oral prednisone at 10mg/day for three months with a progressively descending dose until reaching 5mg/48 hours as a maintenance dose, which was sustained for 18 months. The clinical evolution and progress of laboratory parameters (ACE of 36U/l in September 2010) were satisfactory; the patient had improved cognitive state and increased iPTH levels, with normalised hypercalcaemia. The patient is currently on an HD regimen.
Multiple causes can explain hypercalcaemia in a patient on HD (the use of calcium chelating agents, dialysed with a high-calcium bath, use of vitamin D and derivatives, poorly controlled secondary hyperthyroidism, ABD, and osteomalacia). One uncommon cause is sarcoidosis, a granulomatous disease of unknown origin, in which an extra-renal synthesis of calcitriol is produced by activated macrophages.1 The disease normally involves constitutional symptoms (weight loss, fever), arthralgia, asthenia, and pulmonary symptoms (reticular opacities and bilateral pulmonary hilar lymph node). It tends to respond to corticosteroid therapy that should be maintained for at least one year. Extra-thoracic symptoms occur in only 30% of cases. Cases mentioned in HD patients are rare in the medical literature2-5 and tend to be diagnosed based on hypercalcaemia with systemic and pulmonary symptoms. Our patient had increased ACE levels (75%), neurological symptoms (5%), hepatosplenomegaly (20%-25%), and hypercalcaemia (10%-20%). We had previously observed reticular pulmonary opacities, with no increase in ACE levels or symptoms in other areas that would suggest this diagnosis. Additionally, the patient, being diabetic, had widespread calcifications and multiple risk factors that could explain the vascular encephalopathy; the chronic pneumopathy had already been considered as ABD. The patient’s clinical evolution was latent and progressive, with no elevations in ACE levels or true hypercalcaemia until three years after the first crisis. The non-specific constitutional symptoms and the sum of causes that all could have explained the patient’s condition hindered making the proper diagnosis. After treatment, iPTH levels were stable between 180pg/m and 270pg/m, which was surprising. We also want to bring attention to the fact that cases of hypercalcaemia right on the limit are not always due to ABD, which we believe to be adequate reason to consider other possible diagnoses when the circumstances call for it.
Conflicts of interest
The authors have no conflicts of interest to declare.
Figure 1. Evolution of calcium, phosphorous and intact parathyroid hormone (iPTH) levels
Figure 2. Chest computed tomography. Hilar lymph nodes