Los fibratos representan uno de los grupos de fármacos indicados para el tratamiento de la hiperlipidemia. Uno de sus efectos secundarios, aún poco conocido, es el deterioro agudo de la función renal. En los últimos 26 meses hemos objetivado en nuestra consulta externa de Nefrología un total de 13 pacientes con deterioro de la función renal asociado al uso de fibratos. Material y métodos: El objetivo de nuestro estudio es evaluar nuestra experiencia en el incremento de Creatinina sérica (Crs) inducido por fibratos. Se trata de una revisión retrospectiva de una serie de casos. Resultados: De los 13 pacientes (8 hombres/5 mujeres) con edad media de 65,5 ± 12,2 años, diez fueron tratados con fenofibrato, uno con bezafibrato y dos con gemfibrozilo. Seis pacientes partían de una función renal normal y los otros siete presentaban una Insuficiencia Renal Crónica (IRC) leve-moderada previamente al inicio del tratamiento. El incremento de creatinina con respecto a la basal expresado en porcentaje fue superior al 74%. En nueve de los pacientes el deterioro de función renal fue completamente reversible (grupo 1), mientras que en cuatro de ellos la recuperación fue parcial (grupo 2). La media de creatinina antes de recibir tratamiento con fibratos fue de 1,33 ± 0,36 mg/dl (aclaramiento de creatinina 63,2 ± 26,6 ml/min) y la media de la creatinina máxima durante el tratamiento fue de 2,22 ± 0,49 mg/dl (aclaramiento de creatinina 33,4 ± 8,1 ml/min). El tiempo medio de evolución hasta objetivarse el incremento de creatinina fue de 6,7 ± 5,8 meses y la recuperación de la función renal ocurrió a los 3,8 ± 3,5 meses de la suspensión del tratamiento con fibratos. En los pacientes del grupo 2 se objetivó un mayor incremento de Crs y un tiempo de tratamiento con fibratos más prolongado. En los pacientes en los que se obtuvieron niveles de CPK, éstos fueron normales. En dos de nuestros pacientes se realizaron biopsias renales sin objetivarse alteraciones significativas. Conclusiones: El tratamiento con fibratos puede inducir un deterioro de función renal. En el 30% de los casos de nuestra serie, el aumento de creatinina sólo fue parcialmente reversible tras la suspensión del fibrato. En todos los pacientes que se inicie tratamiento con fibratos se debe monitorizar la función renal con especial atención en aquellos pacientes con cierto grado de insuficiencia renal previa.
Fibrates represent one of the medications used to treat patients with hyperlipemia. Deterioration in renal function is not a very known adverse effect of fibric acid derivates. In the last 26 months we have detected thirteen patients with acute renal failure associated to fibrates in our outpatients’ clinic. Subjects and methods: The aim of our study is to analyze our experience in deterioration in renal function associated to fibrates use. This is a retrospective charts review. Results: From the thirteen patients (8 males/5 females) with mean age of 65.5 ± 12.2 years, ten received Fenofibrate (FN), one Bezafibrate (BZ) and two Gemfibrozil (GF). Six cases had previously normal renal function and the seven remaining had mild chronic renal failure (CRF). The increase of serum Creatinine (Crs) value was higher than 74%. Acute renal failure was reversible in 9 patients (group 1), but the other 4 did not recover their previous renal function (group 2). The average of Crs before fibrate treatment was 1.33 ± 0.36 mg/dl (Creatinine clearance 63.2 ± 26.6 ml/min) and the highest average of Crs during the treatment was 2.22 ± 0.49 mg/d (Creatinine clearance 37.3 ± 11.9 ml/min). The average time until acute renal failure diagnosis was 6.7 ± 5.8 months and the recovery of renal function was delayed an average of 3.8 ± 3.5 months after fibrates withdrawn. Group 2 patients had a higuer Crs and longer time with fibrates than group 1 patients. CPK values were normal in all cases. In two patients renal biopsy was performed and no significant lesions were detected. Conclusion: The fibrate treatment can induce an acute renal failure. Four patients (30.8%) did not recover their basal renal function. When fibrate treatment begins a renal function should be monitored specially in patients with CRF.ABSTRACT Introduction: Fibrates represent one of the medications used to treat patients with hyperlipemia. Deterioration in renal function is not a very known adverse effect of fibric acid derivates. In the last 26 months we have detected thirteen patients with acute renal failure associated to fibrates in our outpatients’ clinic. Subjects and methods: The aim of our study is to analyze our experience in deterioration in renal function associated to fibrates use. This is a retrospective charts review. Results: From the thirteen patients (8 males/5 females) with mean age of 65.5 ± 12.2 years, ten received Fenofibrate (FN), one Bezafibrate (BZ) and two Gemfibrozil (GF). Six cases had previously normal renal function and the seven remaining had mild chronic renal failure (CRF). The increase of serum Creatinine (Crs) value was higher than 74%. Acute renal failure was reversible in 9 patients (group 1), but the other 4 did not recover their previous renal function (group 2). The average of Crs before fibrate treatment was 1.33 ± 0.36 mg/dl (Creatinine clearance 63.2 ± 26.6 ml/min) and the highest average of Crs during the treatment was 2.22 ± 0.49 mg/d (Creatinine clearance 37.3 ± 11.9 ml/min). The average time until acute renal failure diagnosis was 6.7 ± 5.8 months and the recovery of renal function was delayed an average of 3.8 ± 3.5 months after fibrates withdrawn. Group 2 patients had a higuer Crs and longer time with fibrates than group 1 patients. CPK values were normal in all cases. In two patients renal biopsy was performed and no significant lesions were detected. Conclusion: The fibrate treatment can induce an acute renal failure. Four patients (30.8%) did not recover their basal renal function. When fibrate treatment begins a renal function should be monitored specially in patients with CRF.
INTRODUCTION
Dyslipidaemia commonly affects both the general population and patients with CRF.1 Several strategies and hypolipaemiant agents have been employed in the treatment of this condition, including fibric acid derivatives. Fibric acid derivatives are especially recommended for those patients with hypertriglyceridaemia and low HDL cholesterol levels, regardless of the presence of CRF.2,3 The most common side effects associated with fibrates are gastrointestinal and muscular complaints.4 However, different studies have indicated an increase in serum creatinine following the use the of these agents, which calls into question the use of fibrates in patients with altered renal function.5-10 The mechanism that causes this reaction is still unknown. Different hypotheses and arguments have been put forward, however none have been fully proven.
The first patient with increased serum creatinine levels caused by fibrate was diagnosed in our department over two years ago. Since then, another twelve cases have been identified. The aim of this study is to analyse these cases and review the literature on the physiopathology of this phenomenon.
MATERIAL AND METHODS
From March 2006 to May 2008 thirteen patients were identified in the Department of Nephrology as having high serum creatinine levels after initiating treatment with the three fibrates that are commercially available in Spain (fenofibrate, bezafibrate and gemfibrozil). The diagnosis of increased serum creatinine associated with fibrate treatment was made in patients that met the following criteria: 1) an increase in creatinine of at least 20% compared to baseline levels; 2) temporal relationship between the increase in creatinine and fibrate treatment; 3) no other concomitant nephrotoxic medications were being used; 4) all other causes of deterioration of renal function had been reasonably ruled out; and 5) serum creatinine levels improved once the medication was suspended. The patients that met these criteria are the subject of this retrospective review.
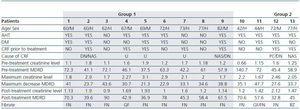
Clinical data was collected from all the patients, including age, sex, medical history, the presence of CRF (defined as SCr > 1.2mg/dl and/or MDRD values < 60ml/min) and its cause. The type of fibrate and the use of concomitant medications if applicable were also included; the use of potentially nephrotoxic agents was of particular interest. The analytical data collected included SCr, estimated glomerular filtration rate using MDRD, proteinuria, triglycerides, cholesterol and CPK whenever possible (76.9% of patients). The variables of all the cases were analysed and afterwards patients were divided into two comparative groups. Group 1: patients that fully recovered baseline creatinine levels (cases 1-9); group 2: patients with partial recovery of renal function (cases 10-13).
The numerical values are expressed as the mean ± standard deviation and the comparisons were carried out using nonparametric tests for paired and unpaired samples. A p < 0.05 value was considered significant.
RESULTS
The mean age of the thirteen patients (eight men and five women) was 65.5 ± 12.2 years. The presence of CRF before initiating fibrate treatment was detected in seven patients (53.8%). None of our patients had undergone a kidney transplant. Their clinical characteristics, including the causes of CRF can be found in table 1.
The fibrates given to our patients included fenofibrate (eleven patients), gemfibrozil (two patients, one of which was previously treated with fenofibrate) and bezafibrate (one patient). With regard to concomitant treatments, nine of our patients were being treated with renin-angiotensin system blocking agents (ACEIs and/or ARBs), however all patients were receiving these treatments regularly before experiencing deterioration of renal function, except one patient who started treatment afterwards. This medication was not suspended in any of the cases. Only two patients received combined treatment with statins and high levels of CPK were not observed in either of them.
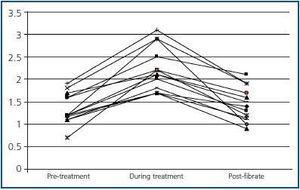
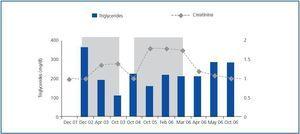
Before fibrate treatment SCr was 1.33 ± 0.36mg/dl, and MDRD was 63.2 ± 26.6ml/min, compared with the maximum creatinine level during treatment which was 2.22 ± 0.49mg/dl, and MDRD which fell to 33.4 ± 8.1ml/min (p <0.05). After suspending fibrate treatment SCr fell to 1.45 ± 0.38mg/dl, and MDRD increased to 58.8 ± 16.6ml/min (p <0.05 in comparison with the maximum SCr value and little difference was observed when compared with baseline SCr levels) (figure 1). The average percentage increase of serum creatinine was 74.6 ± 55.8%. None of the patients required renal replacement treatment. One of the patients presented two episodes of increased SCr during the two periods throughout the follow up and fibrates were recommended as shown in figure 2.
The mean time elapsed until an increase in creatinine observed was 6.7 ± 5.8 months after initiating treatment, while patients experienced a recovery of renal function after 3.8 ± 3.5 months following the suspension of fibrate treatment.
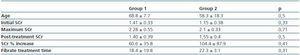
Four patients (30.8%) did not fully recover renal function (group 2: cases 10-13) and two of them did not present CRF before initiating fibrate treatment. The SCr percentage increase of these four patients was 104.4 ± 87.9% compared with an increase of 60.6 ± 35.8% in patients who did fully recover renal function (group 1: cases 1-9). Patients underwent fibrate treatment for 19.8 ± 15.5 months. In the comparison of both groups, the patients in group one received treatment for an average of 18.4 ± 19.8 months compared with the patients in group 2 who received treatment over a period of 22.3 ± 3.1 months (table 2).
With regard to proteinuria, there were no differences in the values before and during the fibrate treatment period. Mean proteinuria before starting treatment was 0.89 ± 1.4g/day, and during fibrate treatment was 0.84 ± 1.1g/day. In those cases where it was possible to obtain CPK levels, these were normal.
A kidney biopsy was carried out on two patients and no alterations that could justify an increase in SCr were identified in either of the cases.
DISCUSSION
This study highlights our experience regarding fibrateinduced deterioration of renal function. Our sample is the second largest of all the studies that have been published up until now. It is also worth highlighting that all thirteen patients were diagnosed within a period of only 26 months, which indirectly implies a high incidence of this phenomenon and further emphasises its importance.
Although the increase in creatinine levels experienced by our patients was mainly associated with fenofibrate, it was also observed in patients treated with bezafibrate and gemfibrozil. These findings do not support the theory put forward by Broeders et al., that states that gemfibrozil is the only fibrate that does not alter renal function.6 Despite this, the creatinine percentage increase was higher in the group of patients treated with fenofibrate compared with the gemfibrozil group (82.1 ± 65.2% versus 38.7 ± 4.1%), although the difference was not significant. Moreover, of the two patients from the gemfibrozil group, one patient had already been treated with fenofibrate. Both these findings could indicate the less deleterious effect of gemfibrozil. The hypothesis that has been put forward to explain this highlights the reduced stimulating effect of gemfibrozil on peroxisome proliferator activated receptors (PPAR-α), which are responsible for inhibiting the synthesis of vasodilating prostaglandins.11
Our patients recorded a mean increase in SCr levels of 74.6 ± 55.8% and a reduction in MDRD following fibrate treatment. These results can be added to those already described by other authors, although the percentage increase is higher in our group of patients in comparison with the papers reviewed, which could be linked to the increased duration of fibrate treatment.5-10 Urea or cystatin C levels were not recorded in this series, however when other authors have recorded these values an increase in both has been observed in correlation with SCr.6,7,9,10 These results suggest that there is a reduction in glomerular filtration as a result of fibrate treatment. However, in the studies carried out by Hottelart et al. and Ansquer et al.10,12 which analysed renal plasma flow and glomerular filtration using paraaminohippurate (PAH) and insulin clearance in patients receiving fibrate treatment, no significant changes were observed. A possible explanation for this could be the short duration of treatment in both studies (two and six weeks).
Like other studies, our series involved patients with normal renal function prior to treatment as well as patients with different grades of CRF.6,7 No studies have been carried out involving the general population or patients with CRF prior to treatment in order to establish the incidence of this phenomenon. A 60% increase in SCr has been obtained from more vulnerable study groups like those that include kidney transplant patients treated with fibrates.6 This is a highly significant finding given that irreversible increases in SCr have been identified specifically in kidney transplant patients after medication is suspended. In our experience, 30.8% of patients did not fully recover their previous renal function. None of our patients had undergone a kidney transplant and 50% of those who did not fully recover their creatinine values had normal renal function. Two factors that could have contributed to causing this irreversible damage are the percentage increase of SCr (104.4 ± 87.9% in cases 10-13 compared with 60.6 ± 35.8% in patients that fully recovered renal function) and the duration of fibrate treatment, which was also longer in the group of patients who only experienced a partial recovery of their renal function (22.3 ± 3.1 months) compared with those whose creatinine increase was completely reversible (18.4 ± 19.8 months), although the differences were not statistically significant. These findings have not been reported in any other series that have already been published, however when the patient data from the Broeders et al. study was analysed a similar trend was identified. This suggests that patients with a greater increase in SCr are less likely to fully recover renal function.
The mechanism implicated in this apparent deterioration of renal function following fibrate treatment has not yet been clearly defined. There are many theories that attempt to explain it; however none have been fully proven.
The most widely accepted hypothesis is based on the haemodynamic changes that fibrates may cause. Fibric acid derivatives are known for stimulating PPAR-α which can lead to reduced renal plasma flow.12-14 This supports the theory that increased creatinine is triggered by haemodynamic factors. However, when the renal plasma flow has been studied using PAH clearance,10,11 no statistically significant changes have been observed that support this theory. In the study by Ansquer et al. which analyses the effect of fibrate treatment over a period of six weeks in patients with normal renal function prior to treatment, a reduction in PAH clearance is observed in the group that received treatment although it is not significant (p = 0.05).15 However, this result could indicate a real reduction in the renal plasma flow that does not reach statistical significance because of the limited number of patients and the short period of time analysed.
Another hypothesis put forward by Hottelart et al., is the increase in serum creatinine as a result of an increase in endogenous creatinine production.10 Several papers have been published that describe increases in SCr without altered creatinine clearance.10,11,16,17 On the basis of this, the authors conclude that there should be an increase in endogenous creatinine production, presumably from muscle, given that muscular toxicity is associated with this group of drugs. However, this theory does not explain the increase in urea or cystatin C which has been identified in several studies.
Following on from this point, rhabdomyolisis could be considered a cause of increased creatinine. Many studies have been published describing episodes of rhabdomyolisis following treatment, either with fibrate treatment alone or combined with statins.18-20 CPK levels were analysed and were always within the normal range for most patients in this study (76.9%). In those cases in this study which involved a kidney biopsy, myoglobin in the renal tubules was not detected.
There is a fourth hypothesis which is defended in the study by ¡ngeles et al. which analyses the histopathological results from the biopsies of three transplant patients who experienced a reversible increase in creatinine levels following treatment with fenofibrate.9 The kidney biopsies showed toxic tubular damage in the form of cytoplasmic degeneration affecting the proximal tubule cells and the presence of prominent hyaline granules. These findings do not coincide with those obtained from the biopsies of the two patients in our series which did not show any pathological lesions.
Finally, in the case of transplant patients undergoing treatment with calcineurin inhibitors, several authors have highlighted the deleterious effects of combining this group of immunosuppressants with fibrates. Different studies have identified an increase in cyclosporin levels which could contribute to nephrotoxicity.21 The possible role of fibrates in enhancing the nephrotoxicity of calcineurin inhibitors without altering serum levels has also been suggested. Regardless of the mechanism, this could explain the high incidence of increased creatinine levels as a result of fibrate treatment identified in transplant patients.
We believe, on the basis of our own experience, that a fibrate-induced increase in SCr may be the result of haemodynamic factors. Deterioration of renal function is potentially reversible, however if the duration of fibrate treatment is too long this could result in permanent kidney damage.
To conclude, fibrate treatment may induce an increase in SCr and the deterioration of renal function. This is particularly relevant when dealing with patients with CRF since, if during the follow up they experience deterioration of renal function, the possibility of increased creatinine associated with fibrate treatment should be weighed up against the possibility of disease progression. The physiopathology of this phenomenon is not yet known, however it is becoming increasingly relevant given that in over 30% of the cases in our series, the increase in creatinine levels was not completely reversible. The renal function of all patients who undergo fibrate treatment should be monitored and patients with a significant degree of renal failure prior to treatment require special attention. If an increase SCr levels is detected, treatment should be suspended.
Figure 1.
Table 1. Clinical and analytical patient characteristics Group 1: Complete recovery of renal function. Group 2: Partial recovery of renal function
Figure 2.
Table 2. Comparison of the groups with complete recovery (group 1) or partial recovery (group 2) of renal function