Desde su introducción en la década de los 70, la evolución de los calcioantagonistas ha permitido solventar la incertidumbre inicialmente generada por aquellos fármacos de primera generación. Éstos se caracterizan por una menor disponibilidad oral, una acción vasodilatadora rápida y corta acción. El manidipino surge como un derivado dihidropiridínico de tercera generación con ventajas adicionales reales frente a las generaciones anteriores, como son su alta lipofilicidad, su acción más prolongada y una vida media prolongada a nivel del receptor y, además, algunas ventajas teóricas entre otras, mejoras sobre la función renal reduciendo la presión intraglomerular y la microalbuminuria. Sin embargo, la evaluación clínica de estas últimas propiedades depende aún de los resultados que se deriven de la experiencia clínica. Además de ahondar en su papel en la reducción de la presión arterial, presentamos una breve revisión sobre nuevos aspectos cardiometabólicos de los calcioantagonistas dihidropiridínicos, centrándonos en el manidipino.
From its introduction in the decade of the 70’s the evolution of the calcium channel blockers has allowed to resolve the uncertainty initially generated by those first generation drugs. These, are characterized by a smaller oral availability, a fast vasodilator action and a short duration of action. Manidipine arises as a dihydropyridine calcium antagonist of third generation with real additional advantages regarding to previous generations. They show high lipophilia, a more prolonged action and as well as a prolonged average life at the level of his receptor and, in addition, some theoretical advantages among others calcium antagonists, improvements on the renal function by reducing the intraglomerular pressure and microalbuminuria. Nevertheless, the clinical evaluation of these last properties still depends on the results derived from clinical trials. Besides to go deep in its role in their antihypertensive effect, we presented a brief review on new cardiometabolic aspects of these dihydropyridines calcium antagonists focusing in manidipine.
INTRODUCTION
Hypertension and insulin resistance are the most important risk factors for cardiovascular damage.1,2 Antihypertensive treatment commonly includes the combination of calcium channel antagonists (CA) with angiotensin converting enzyme inhibitors (ACE inhibitors) or angiotensin II receptor blockers (ARBs),3 since single-agent treatment is not able to reduce blood pressure in most cases.
Some studies4,5 show that anti-hypertensive treatments that include calcium antagonists improve insulin sensitivity. In a study of 158 hypertensive patients with type-2 diabetes, the combination of manidipine and delapril showed results similar to those of olmesartan and hydrochlorothiazide as far as reducing arterial pressure, but with fewer changes in orthostatic arterial pressure, as well as fewer adverse metabolic effects. These data are more interesting for this group of patients, which is especially prone to orthostatic hypotension associated with an increase in morbidity and mortality.5
Preliminary studies of diabetic patients with uncontrolled hypertension and microalbuminuria suggest that, despite adequate treatment with ACE inhibitors or ARBs, manidipine may be given in combination with the renin-angiotensin inhibitors to normalise blood pressure and urinary excretion of albumin in diabetic patients.6
Calcium antagonists constitute a highly heterogeneous class of molecules that may be grouped into phenylalkylamine derivatives, such as verapamil, benzothiazepine derivatives whose prototype is diltiazem, and 1,4-dihydropyridine compounds that include manidipine.7
The first generation of dihydropyridine calcium channel blockers, such as nifedipine, are characterised by their instant release, their short half-life and their rapid absorption. Despite having a favourable metabolic profile, they present some adverse effects, such as sudden drops in arterial pressure, tachycardia and sympathetic activation. In the second generation, that includes amongst others, felodipine and isradipine is characterised by a slower molecular release.8
The latest generation of calcium antagonists, with a long half-life and extended duration of action has shown a clear decrease in arterial pressure and a significant reduction in side effects. On this subject, a trial with 30 obese hypertensive patients treated with amlodipine, manidipine and cilnidipine revealed that these long-acting calcium antagonists reduce arterial pressure, and in addition, reduce insulin resistance, suggesting important cardio-metabolic properties.4
VOLTAGE-DEPENDENT CALCIUM CHANNELS
Voltage-dependent calcium channels are classified according to their pharmacological and electrophysiological properties as L, P/Q, N, R and T-type.9 They are composed of multiple heteromeric subunits: α, β, γ δ. The α1 subunit seems to be responsible for the prinicipal characteristics of these channels.10-12
Unlike the first calcium antagonists that only blocked L-type channels, third-generation blockers, such as manidipine, lercadipine, amlodipine, nivaldipine or efonidipine, are capable of blocking both L-type and T-type channels, the latter are only active during cellular proliferation.
L-type channels are activated by intense depolarisations, resulting in a prolonged calcium influx in a large variety of cell types. In this way, they play a central role in the contraction of cardiac and smooth muscle cells (Cav1.2[α1C]), are responsible for arterial smooth muscle tone, and have become pharmacological targets in the treatment of hypertension and angina. In the kidney, (Cav1.2[α1C] and Cav1.3[α1D]) they promote the dilatation of pre-glomerular or afferent arterioles, increasing glomerular hypertension by a considerably. Furthermore, we find other L-type channels in skeletal muscle Cav1.1(α1S), brain and kidney (Cav1.2[α1C], Cav1.3[α1D]), pancreas (Cav1.3[α1D]) and retina (Cav1.4[α1F]).9
T-channels are expressed in the nervous system (Cav3.1[α1G]), brain (Cav3.1[α1G], Cav3.2[α1H] and Cav3.3[α1D]), heart (Cav3.2[α1H]), kidney (Cav3.1[α1G], Cav3.2[α1H]) and liver (Cav3.2[α1H]).9 Their influence in hormone secretion such as renin, aldosterone, atrial natriuretic peptide and insulin has been suggested in multiple occasions. T-type channels are activated by short depolarisations, which provoke a transitory flow of calcium. In post-glomerular or efferent arterioles, only T-type channels and no L-type channels are expressed and this implies that their tone must be controlled by T channels and AT1 angiotensin II receptors. Manidipine and lercanidipine block T-type channels in efferent arterioles, thus diminishing the intraglomerular pressure and also the excretion of albumin; at the same time, they block L-type channels, favouring afferent arteriole dilation. In this way, T-type channel antagonists influence renal haemodynamics through their anti-hypertensive action.9,13-15 We can therefore consider their effect to be protective against renal damage, since the kidney is one of the targets for end-organ damage in hypertensive and diabetic patients.
Non-haemodynamic action of T-type channel antagonists may have multiple beneficial effects by inhibiting inflammatory processes (inhibition of Rho kinase, NF-kB, leukocyte adhesion) and blocking the renin-angiotensin system and the sympathetic nervous system.13
Apart from L and T-type channels, P/Q type channels are found in the brain, kidneys and pituitary gland ([Cav2.1(α1A)], N-type channels are found in the brain and nervous system (Cav2.2[α1B]) and R-type channels in the brain, heart and pituitary gland (Cav2.3[α1E]).9
MANIDIPINE, ADIPOGENESIS AND INSULIN SENSITIVITY
The lipotoxicity hypothesis suggests that in type-2 diabetes the adipose tissue looses the ability to accommodate an excess of calories. The loss of adipocyte differentiation causes the excess of calories to accumulate, mainly in the liver, pancreas and muscle tissue, which contributes to the development of insulin resistance.16 Small adipocytes are sensitive to insulin, unlike the mature ones, which become hypertrophied and insulin-resistant.Therefore, favouring adipogenesis will contribute to lowering insulin resistance in type-2 diabetics.
The improvement of insulin sensitivity gained with dihydropyridine calcium antagonists is almost imperceptible.
Nifedipine, only blocks L-type channels, worsens insulin resistance and inhibits the release of glucose.4 However, studies with manidipine show surprising results in this respect. It has already been shown in a randomised clinical trial of 64 hypertensive patients with metabolic syndrome, evaluated according to NCEP/ATPIII criteria, treated with manidipine or amlodipine during 12 weeks that although similar decreases in arterial pressure were observed with both treatments, only the patients treated with manidipine show a significant reduction in insulin resistance.17 Similarly, the most recent analysis of the effects of the combination of delapril/manidipine compared with olmesartan/hydrochlorothiazide on insulin sensitivity and plasma fibrinogen in obese hypertensive patients showed that the first combination significantly reduced insulin resistance and plasma fibrinogen levels, despite the fact that the decrease in arterial pressure values was similar for both combinations.18
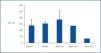
Studies by others have shown that manidipine, but not amlodipine or lercadipine, activates the peroxisome proliferator-activated receptor (PPARγ) in 3T3-L1 adipocytes in the rat.19,20 In our studies of NIH-3T3 cells treated with manidipine, we observed an increase in the expression of both PPARγ and the adipocyte differentiation 2 (aP2) gene. The latter is an indicator of adipogenesis and possibly expressed after induction of the former (figure 1). These results suggest that manidipine increases insulin sensitivity in hypertensive diabetic patients by stimulating the formation of new adipocytes (unpublished observations). In addition it has been observed that the increase in intracellular calcium inhibits pre-adipocyte differentiation.21 This is distinct from the well known role of calcium in more rapid processes, such as neurosecretion, excitation or contraction.
Adipogenesis, like other differentiation processes, depends on transcription factor promoters such as PPARγ and transcription factor inhibitors, such as the GATA family, which in turn are activated by extracellular signals. Calcium homeostasis was studied with particular attention to the calreticulin, the principal protein that binds to calcium in the lumen of the endoplasmic reticulum, and which is largely responsible for rapid calcium exchange. A study of stem cells and 3T3-L1 pre-adipocytes shows that calreticulin could modulate adipogenesis by means of a negative feedback mechanism. PPARγ is a potent calreticulin transcription activator, since it binds to its promoter. In this way, it increases calreticulin expression, but once calreticulin is over-expressed, it inhibits cis binding of the PPARγ-RxR heterodimer to PPARγ response elements (PPRE), cancelling transcriptional activation of PPARγ by fatty acids. By this mechanism, calreticulin negatively regulates both the expression of PPARγ and other critical pro-adipogenic transcription factors such as C/EBPα.22
The action of manidipine as a calcium channel blocker may prevent calcium from entering the cell thus, decreasing the concentration of calcium in the endoplasmic reticulum and therefore, the concentration of calreticulin, favouring adipocyte differentiation.
MANIDIPINE: ANTI-INFLAMMATORY, VASOPROTECTIVE AND ANTIOXIDANT EFFECTS
Oxidative stress plays a fundamental role in the development of atherosclerosis. Several studies demonstrate the beneficial effect of dihydropiridine calcium antagonists on atherosclerotic lesions.23 The beneficial effects of calcium blockers in macrovascular endothelial cells should be demonstrated and proved using mechanisms that do not include calcium channels, since these are not expressed in endothelial cells.24 For this reason, some authors have speculated that the action of dihydropyridines on this tissue was related with their lipophilic nature.25 Toba et al. point out the antioxidant, anti-inflammatory effect of manidipine and other third-generation calcium antagonists such as amlodipine, measured by the increase of expression of endothelial nitrous oxide synthase (eNOS) and the inhibition of the expression of angiotensin converter enzyme (ACE) , but not by its possible action of lowering arterial pressure . In this study, we observe that manidipine normalises the decrease in both the eNOS gene and its protein, and attenuates the over-expression of NAPDH oxidase, VCAM and MCP-1 in the aorta of hypertensive rats.26
Furthermore, manidipine shows another beneficial effect on atherogenesis, since it inhibits the expression of LOX-1, a low-density lipoprotein receptor induced by angiotensin II.27
Sun X et al28 recently showed that in differentiated mature adipocytes, 3T3-L1 or co-cultures with both cell types, calcitriol increases expression of inflammatory molecules such as MCP-1, MIF, M-CSF, MIP, IL-6, TNF or CD14. Treatment with nifedipine or dinitrophenol inhibits the action of the calcitriol, which reveals a mechanism that is dependent on calcium and mitochondrial uncoupling. Therefore, it would seem that blocking calcium channels with manidipine would produce an antioxidant, antiinflammatory effect by lowering the intracellular calcium level.
KEY CONCEPTS
1. Voltage-dependent calcium channels are classified according to their pharmacological and electrophysiological properties as L, P/Q, N, R and T-type. They are composed of multiple heteromeric subunits: α, β, γ and δ. The α1 subunit seems to be responsible for principal characteristic of these channels.
2. L-type channels are activated by intense depolarisations that are responsible for the tone of smooth arterial muscle. Ttype channels are activated by short depolarisations, which provoke a transitory flow of calcium.
3. Although the improvement in insulin sensitivity achieved with dihydropyridine calcium antagonists is almost imperceptible, we have observed an increase in the relative expression of PPARγ (peroxisome proliferator-activated receptor) and the relative expression of the adipocyte differentiation 2 (aP2) gene. These preliminary results may suggest that manidipine increases insulin sensitivity in hypertensive diabetic patients by stimulating the formation of new adipocytes and preserving PPARγ activity.
Figure 1.