Las fosfatoninas son factores reguladores del metabolismo del fósforo, y el FGF23 es el mejor estudiado de ellos. Esto ha producido un cambio en nuestra comprensión del metabolismo mineral, y especialmente de la regulación del fósforo. El FGF23 es un factor de 251 aminoácidos con una novedosa porción carboxilo terminal de 71 aminoácidos, producido primariamente por los osteocitos en el hueso. Tiene un rol central en la regulación de la homeostasis del fósforo, produciendo fosfaturia, y de la vitamina D, inhibiendo su producción por supresión de la 1 alfa hidroxilasa renal. Se ha pensado que tiene un papel importante en la patogénesis del hiperparatiroidismo secundario temprano relacionado a la insuficiencia renal crónica al inhibir la síntesis renal de 1,25(OH)2D en respuesta a su incremento en sangre producido para favorecer la excreción renal de fósforo y mantener su balance. En IRC, sus niveles parecerían ser predictores independientes de progresión hacia la IRC terminal. En los pacientes en diálisis, sus niveles permitirían predecir el resultado de la terapia con calcitriol, así como parecen ser predictores independientes de riesgo de mortalidad en el primer año de hemodiálisis. Sus niveles también se han relacionado con el desarrollo de calcificaciones vasculares en arterias de las manos, pero con las calcificaciones aórticas. La exposición a niveles excesivos de FGF23 en período temprano postrasplante parece estar más fuertemente asociado con la hipofosfatemia postrasplante que la PTH.
Phosphatonins are regulatory factors of phosphate metabolism and the FGF23 is the best studied of them. This has produced a change in our understanding in mineral metabolism and specifically of phosphate regulation. FGF23 is a 251-amino acid factor that differs from other FGF family members by having a 71-amino acid extension on the carboxyl-terminal end of the molecule that is specific for this factor. It is primarily produced by osteocytes in bone. It has a central role in phosphate homeostasis regulation, producing phosphaturia, and in vitamin D metabolism, inhibiting its production by suppression of renal 1 Alfa hydroxylase. It is believed to have an important place in the pathogenesis of early secondary hyperparathiroidism related to chronic renal insufficiency by inhibiting renal synthesis of 1,25(OH)2D in response to its increment in blood produced to increase renal phosphate excretion and maintain phosphate balance. In CRF its serum levels seem to be independent predictors of progression to terminal renal failure. In dialysis patients the determination of its serum levels would allow to predict the results of therapy with calcitriol in the treatment of secondary hyperparathyroidism; they also seem to be independent predictors of the risk of mortality during the first year of hemodialysis. Its serum levels have also been related to the development of vascular calcifications of hand arteries but not with aortic calcifications. The exposure to excessive levels of FGF23 in the early postransplant period seems to be strongly associated with postransplant hypophophatemia more than to PTH or other phosphatonins.
INTRODUCTION
The recent discovery of the phosphatonins as regulatory factors of the phosphate metabolism,1 especially of FGF23,2 the most extensively studied, has expanded our understanding of mineral metabolism led us question former hypotheses.
According to the classic paradigm, the regulation of serum inorganic phosphate (Pi) is controlled by variations in two key calciotropic hormones.3
1. Increases in extracellular phosphate directly stimulates PTH secretion and this in turn inhibits the renal tubular reabsorption of phosphate.
2. Increases in extracellular Pi reduces the expression of renal 1-Alfa-hydroxylase thereby reducing the synthesis of 1.25(OH)2 D3. This results in a decrease in the intestinal absorption of Pi.
However, in a group of pathological conditions, alterations in phosphate levels were observed without significant modifications in PTH or 1.25(OH)2 D3 concentrations. This can not be explained by the classic pathways of phosphate regulation, suggesting that there might be other factors involved in its regulation.
These diseases that are characterised by hypophosphataemia, hyperphosphaturia and defective bone mineralisation include the X-linked hypophosphataemia (XLH),4 autosomal dominant hypophosphataemic rickets (ADHR)5 and its recessive form (ARHR)6 and tumour-induced osteomalacia.7
The evidence in favour of the existence of other circulating factors that regulate the levels of serum Pi came from:
1. The oncogenic osteomalacia syndrome: Small mesenchymal tumours that cause renal loss of phosphate and osteomalacia, all of which disappear with the resection of the tumour, suggesting a humoural base for this phenomena.8,9
2. The Hyp mouse, an animal model of the XLH disorder. Renal loss of phosphate can be induced by performing a parabiosis between a normal mouse and a Hyp10 mouse and in a kidney of a normal mouse when transplanting it in a Hyp mouse. Both experimental models also suggest the presence of a circulating factor responsible for the phosphaturia.11
The fibroblast growth factor 23 (FGF23) is a 251-amino acid protein with a molecular weight of 32 Kda belonging to the 22 fibroblast growth factor family. It consists of a signal peptide with 25 amino acids in the amino-terminal domain, a FGF homology region shared by the other 22 fibroblast growth factor family and a novel 71-amino acid carboxylterminus. Between the latter two domains there is a cleavage site that contains an RXXR motif. This motif is characteristic of the cleavage sites for enzymes of the proconvertase-type of of the subtilisin/kexine type of the serine protease family. It is probable that the proconvertases are the enzymes responsible for the degradation of the FGF23. The cleavage site is found mutated in patients that are carriers of ADHR.12 The FGF23 is primarily produced by the osteocytes in bones.13,14
The FGF have four types of receptors: FGFR 1, 2, 3 and 4, with subtypes. FGF23 binds with high affinity to the FGFR1 (IIIC) which is expressed by renal tubular cells,15 primarily in the distal tubule, while FGFR3, the main FGF receptor, is expressed by the proximal tubule, the site of phosphate regulation. In addition, FGF 23 requires for its signaling a transmembrane protein known as Klotho.16,17 The Klotho protein binds to multiple FGF receptors, increasing their affinity to FGF23. The binding to Klotho occurs at the carboxyl terminus. The expression of Klotho in a tissue determines if the tissue is a potential target for FGF23 or not.
The study of the phenotype of the null mice for FGF23 indicated that the FGF23 has a central role in the phosphate and vitamin D homeostasis.18 Heterozygote mice were completely normal, with average serum levels of FGF23, but those mice where both alleles for the FGF23 gene were mutated had a characteristic phenotype with extreme hyperphosphataemia and high serum levels of 1.25(OH)2D. They developed hyperphosphataemia because of the renal retention of phosphate, with an increase in apical sodium/phosphate NaPi-2a co-transporters in the proximal tubule, as well as an increase in the deposition of phosphate in renal histological sections. Those mice deficient in FGF23 also had high levels of 1.25(OH)2D resulting from the increase in the activity of the renal 1·-hydroxylase. The null mice for FGF23 developed progressive hypercalcaemia, presumably from the very high levels of 1.25(OH)2D, and they finally died from renal failure and nephrocalcinosis. The characteristics of the hyperphosphataemia and the increase in 1.25(OH)2 D levels are mirror images of the syndrome caused by the excess of FGF23, and this fits well in the hypothesis that FGF23 is a physiological regulator of Pi and of the vitamin D metabolism.
New strategies have been utilized to study the mechanism of action of FGF23 using monoclonal anti-FGF23 antibodies that can neutralise the activities of the FGF23 in vitro as well as in vivo. Yamazaki et al. developed two antibodies (FN1 and FC1) that recognised the N- and C- terminal regions of the FGF23, respectively.19 Both antibodies inhibited the activity of the FGF23 in a trial based on Klotho-dependent cells. Their administration caused substantial increases in the levels of serum phosphate and 1.25(OH)2 D in normal mice. These changes were accompanied by abnormalities in the expression of the sodium-phosphate NaPi IIA type cotransporter, 25-hydroxyvitamin-D 1alpha-hydroxylase and 24-hydroxylase. Therefore, this study using neutralising antibodies suggests that the FGF23 is a true physiological regulator of the metabolism of phosphate and vitamin D.
FGF23 in early CKD
In a recent large cross-sectional study that included more than 1,800 patients with CKD, Levin et al. demonstrated that the levels of calcitriol started to decrease with the slightest reductions in estimated glomerular filtration rate.20 Some authors have suggested that this calcitriol reduction might be related to a deficit of its substrate, the 25OH Vitamin D. However, Levin did not find a correlation between the two forms of vitamin D: approximately 45% of the patients with low levels of calcitriol had normal levels of 25OHD. In early chronic kidney disease, it has been long established that the decrease in the 1.25(OH)2D levels and the appearance of secondary hyperparathyroidism are produced when there is still no Pi retention and the serum Pi may even be reduced.21 An important question rises from here: Does the FDF23 play an important role in the pathogenesis of the early secondary hyperparathyroidism by inhibiting the renal synthesis of 1.25(OH)2 D in response to the changes in the phosphate handling? And if this is so, how exactly are the changes in Pi are to be detected?
The studies of FDF23 in early CKD are limited due to the difficulties in estimating with precision the GFR in mild renal dysfunction. Also, the C-terminal portion of the FGF23 may be filtered by the kidneys, for which the decrease in glomerular filtration could lead to the accumulation of these fragments of FGF23. It remains unclear if this also happens with the intact molecule of FGF23. Currently, there are two types of tests to measure the FGF23, and both are ELISA assays one detects the Cterminal portion of the factor and usually is reported in RU/ml and the other detects the complete peptide and is reported in pg/ml. Presently, the relationship that exists between both measurements remains unclear, as well as what are the normal ranges in different populations.
Various studies have analysed the relationship between the FGF23, PTH and calcitriol in early CKD. Shigematsu et al. studied 62 pre-dialysis patients with an average age of 51.3 ± 14.0 years (32 men and 30 women).22 They estimated the glomerular filtration rate by 24 hour collections (Ccr) and by measuring cystatin. They divided the patients into three groups according to the Ccr level: those with a Ccr > 80, the second group, Ccr from 80 to 30 and the third group, Ccr < 30ml/min. The serum levels of FGF23 were determined by a double antibody ELISA assay that did not detect biologically inactive N-terminal and C-terminal fragments. These authors found that the serum levels of FGF23 increased exponentially with the decrease of the clearance of creatinine, but that the increase was only significant in the group with a Ccr < 30ml/min. Both the levels of iPTH as well as PTH (1- 84) were tightly correlated with the levels of FGF23, while a negative correlation (also exponential) between the serum concentrations of FGF23 and calcitriol. The maximum rate of tubular reabsorption of phosphate was negatively correlated with the serum concentrations of FGF23. However, the daily quantities of urinary phosphate excreted were significantly lower in patients with a Ccr < 30ml/min, while their circulating levels of FGF23 were significantly higher.
In another study, Gutiérrez O et al. studied 80 patients with CKD and they classified them according to their estimated estimated glomerular filtration rate (eGFR) using the MDRD formula. They determined FGF23 (carboxyl-terminal portion), PTH, 25 (OH)D calcitriol, calcium, phosphate and the fractional excretion of phosphate (FEPi). Various hypotheses were evaluated including the association between FGF23 and decreased calcitriol levels, regardless of renal function, hyperphosphataemia and levels of 25(OH)D using multiple lineal regression. They demonstrated that FGF23 and PTH were inversely associated to eGFR, while the calcitriol levels were lineally associated to the eGFR.
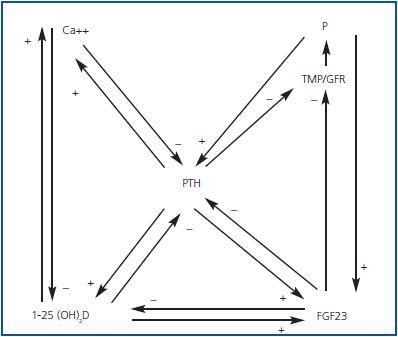
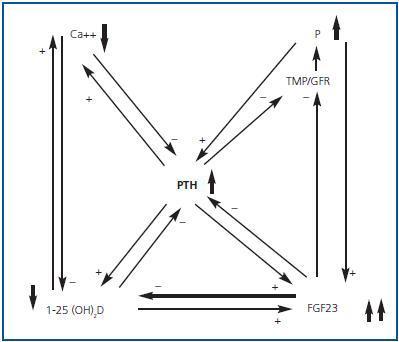
Hyperphosphataemia and hypocalcaemia were found in only 12 and 6% of the patients, respectively, all of which had an eGFR < 30. The increases in the FEPi were associated with decreases in eGFR and increases in both FGF23 and PTH were associated in an independent manner to the increase in FEPi. The increase in the FGF23 and the decrease of the 25(OH)D were independent predictors of the calcitriol decrease. The effects of the renal function and the hyperphosphataemia on the calcitriol levels disappeared completely after adjusting for FGF23. They concluded that the increase in FGF23 could contribute to maintaining the normal levels of phosphate in the presence of CKD at the expense of worsening calcitriol deficiency. Therefore, FGF23 could be a central factor in the early pathogenesis of the secondary PTH (figures 1 and 2).
Recently, the FGF23 has been proposed as a predictor of the progression of renal failure. Fliser et al. studied a cohort of 227 non-diabetic patients with CKD where the plasma concentrations of FGF23 were evaluated with other variables of the phosphorus and calcium metabolism.24 At baseline they found a significant inverse correlation between GFR and the levels of both C-terminal and intact FGF23. 177 of these patients were followed prospectively for an average of 53 months to evaluate the progress of the renal disease. Doubling of the serum creatinine and/or terminal renal failure was observed in 65 patients. By the Cox regression, the C-terminal as well as the intact FGF23 were independent predictors of the progression of CKD after adjusting for age, sex, baseline GFR, proteinuria, Ca, Pi and iPTH. The explanation of the predictive value of the FGF23 in the progression of CKD could come from its inverse relationship with the production of calcitriol and the association between low vitamin D levels and between this and tissue inflammation. A recent study examined the potential role of calcitriol in the inflammation of renal tissue associated with the chronic kidney disease. Zehnder et al.25 measured the vitamin D metabolytes, inflammation markers and gene expression in 174 patients with a variety of kidney diseases. The MCP-1 urinary protein and the renal infiltration by macrophages were each inversely correlated with the serum levels of 1.25(OH)2 D. The analysis of logistic regression with the MCP-1 urinary protein used as a binary outcome showed that for each 10 units increase in the serum levels of 1.25(OH)2 D or 25OHD, there was less renal inflammation. The analysis of 111 renal biopsies showed that renal damage was not associated with a compensating increase in the RNAm for the 25- hydroxyvitamin D-1α-hydroxylase (CYP27B1). In vitro, the 1.25(OH)2 D attenuated the MCP-1 protein expression induced by TNF by the human proximal tubular cells. These data are consistent with the suggestion that renal inflammation is associated to a decrease in vitamin D serum metabolytes.
FGF23 in advanced renal failure
Various studies have evaluated the utility of measuring FGF23 levels in patients with advanced chronic renal disease in various circumstances:
1. As predictors of the results of therapy with calcitriol: Kazama et al. studied 62 patients in haemodialysis with plasma levels of intact PTH greater than 300pg/ml, to which calcitriol had been administered intravenously three times per week.26 The patients whose PTH decreased to less than 300pg/ml within 24 weeks were defined as those treated successfully. The FGF23 was also determined in these same patients with an ELISA assay. The pre-treatment levels of FGF23 were correlated with the intact PTH levels, Ca x Pi product and the history of treatment with active vitamin D. The pretreatment levels of FGF23, iPTH and calcium were lower in patients that had been successfully treated with calcitriol. The logistical regression analysis confirmed that the pre-treatment levels of iPTH and FGF23 significantly affected the results of treatment. The ROC curves showed that the levels of FGF23 were the most useful as as a predictor test to identify patients with a future refractory response to treatment with calcitriol. The treatment has been demonstrated to be successful in 88.2% of those with levels of FGF23 ≤ 9,860ng/l and iPTH ≤ 591pg/ml, while it would be considered satisfactory only in 4.2% of those with values above the mentioned values.26 The investigators concluded that the pre-treatment serum levels of FGF23 were a good predictive indicator of the response to treatment with calcitriol and that their measurement together with the iPTH levels would be important in the follow-up of secondary PTH.
2. As predictors of the risk of mortality in the first year of haemodialysis: Gutiérrez et al. studied the mortality according to serum levels of Pi in a prospective cohort of 10,044 patients that started haemodialysis.27 Later, they analysed the FGF23 levels and the mortality found in a case-control sample of 200 subjects that died and 200 that survived during the first year of HD. Tested the hypothesis that the increased levels of FGF23 when starting haemodialysis could be associated with an increase in mortality. The adjusted multi-variable analysis showed that the increases in the levels of FGF23 were associated with a monotonic increase in the risk of death when they were analysed as continuous variables (odds ratio by unit of increase of the transformed log values of cFGF23: 1.8; CI 95%, 1.4 to 2.4) or in quartiles, with the 1st quartile as a reference: OR for the 2nd quartile, 1.6 (CI, 0.8 to 3.3), OR for the 3rd quartile, 4.5 (CI, 2.2 to 9.4), and OR for the 4th quartile, 5.7 (CI, 2.6 to 12.6). The researchers also found that the predictive value of the elevated serum levels of FGF23 was preserved with different degrees of hyperphosphataemia, except for the highest quartile (> 5.5mg/dl), that was per se associated with a 20% increase in the adjusted risk of death. It is not clear if the increase in mortality is due to cardiovascular causes, which should be the subject of future research. It is not clear either if the FGF23 directly affects cardiovascular function.
3. As predictors of vascular calcifications, Inaba et al. studied the importance of the FGF23 in the development of vascular calcifications.28 These authors investigated male patients with Diabetes Mellitus (DM) and without DM on haemodialysis. The plasma levels of FGF23 were determined at the beginning of the dialysis sessions using ELISA. The vascular calcifications were evaluated by x-rays of the aorta and the arteries of the hands by an examiner, blind to the characteristics of the patients. In non-DM patients, the analysis of the multiple regression showed that the levels of FGF23, the product of Ca x Pi, and body weight were independent factors significantly associated with the calcification of the arteries in hands, and the diastolic blood pressure was associated with the calcification of the aorta. In DM patients, the plasma levels of FGF23 and the duration of haemodialysis were independent factors associated to the calcifications of the hand arteries, while the diastolic blood pressure was associated with aortic calcifications.
In another study, Kojima et al.29 analysed patients on haemodialysis with and without DM. The levels of FGF23 were measured at the start of dialysis by ELISA. The calcifications of the abdominal aorta were evaluated by a computerised tomography and the aortic calcification index was calculated. The authors did not find any relationship between the FGF23 values and aortic calcification.
FGF23 after renal transplantation
Bhan et al.30 carried out a longitudinal prospective study in live-related transplant recipients to evaluate the hypothesis that the excessive levels of FGF23 accounted for the hypophosphataemia and decrease in the levels of calcitriol that follow a renal transplant. The presence of hypophosphataemia < 2.5mg/dl was developed in 85% of the subjects, including one with a previous parathyroidectomy. Hypophosphataemia of ≤ 1.5mg/dl developed in 37% of the subjects. The levels of FGF23 fell quickly within the first week to pre-transplant values of 1,218 ± -542 to 557 ± - 579RU/ml, which were still clearly above the normal values. The FGF23 levels were associated in an independent manner to the serum levels of phosphate (p < 0.01), the urinary excretion of phosphate (p < 0.01) and the calcitriol levels (p < 0.01), while the PTH levels were not associated in an independent manner to any of these parameters. The investigators calculated the area under the curve for FGF23 and PTH for the pre- and first post-transplant measurements to assess the total early exposure to these phosphaturic hormones. An area under the curve for the FGF23 greater than the median was associated with a relative risk to develop hypophosphataemia ≤1.5 mg/dl of 5.3 (p = 0.02), compared with lower levels. On the other hand, the increase in the area under the curve for PTH was not associated with a greater risk of hypophosphataemia. The exposure to excessive levels of FGF23 in the early post-transplant period seems to be more strongly associated with post-transplant hypophosphataemia than PTH.
In another study, Pande et al31 evaluated the levels of two phosphatonins FGF23 and sFRP-4 in patients after renal transplantation. They observed dramatic reductions in the FGF23 (-88.8 ± 5.4%) and in the phosphate (-64 ± 10.2%) 4- 5 after transplantation. In patients with post-transplant hypophosphataemia, the levels of FGF23 were inversely correlated with plasmatic phosphate (r2 = 0.661, p < 0.05), while there was no relationship between the levels of sFRP4 and post-transplant hypophosphataemia.
Conclusions
FGF23 has emerged in the last few years as an important factor in the feedback mechanism that regulate mineral homeostasis, chiefly controlling the excretion of phosphate and the synthesis of calcitriol at the renal tubular level. The recognition that the FGF23 is elevated in early renal failure has stimulated various researchers to study its role in the genesis of secondary hyperparathyroidism, fundamentally because of its primary effect in reducing the production of calcitriol. Since FGF23 is already found in elevated concentrations in terminal chronic kidney disease, different studies have shown the importance of measuring this factor to predict the resistance to treatment with calcitriol in secondary hyperparathyroidism, or in the prediction of early mortality in dialysis. Additional studies in larger cohorts of patients are needed to corroborate these findings. For this reason the measurement of FGF23 in patients with chronic kidney disease has not been included in the guidelines for the management of mineral metabolism in dialysis and transplant patients. It is not clear if the determination of this factor adds more useful information for the clinical management of patients the standard determinations, such as the measurement of serum PTH levels and other parameters of the phosphorus and calcium metabolism.
KEY CONCEPTS
The increase in FGF23 levels may be responsible for the decrease in the levels of calcitriol in early renal failure and it might contribute to the development of secondary hyperparathyroidism of chronic kidney disease.
The predictive value of the determinations of FGF23 for progression to terminal CKD in patients with predialysis CKD has been recently demonstrated.
In patients on dialysis, the determinations of FGF23 may be useful to predict the response to treatment with calcitriol in the management of secondary hyperparathyroidism, as well as to predict the risk of mortality in the first year of haemodialysis and to predict some types of vascular calcifications.
In the early post-transplant period, levels of FGF23 seem to be more strongly associated with post-transplant hypophosphataemia than PTH or other phosphatonin values.
Figure 1.
Figure 2.