Introducción: Las alteraciones en el diafragma de filtración y/o citoesqueleto del podocito están relacionadas con proteinuria y síndrome nefrótico. En nuestra población, la glomerulopatía más frecuente, demostrada por biopsia, es la glomerulosclerosis focal y segmentaria. Nuestro objetivo fue buscar alteraciones en la expresión de algunas de las proteínas asociadas con el diafragma de filtración en pacientes con proteinuria en rango nefrótico. Métodos: Tejido renal de 40 pacientes con proteinuria en rango nefrótico, de 10 pacientes con proteinuria leve, de tres con hematuria aislada y 10 muestras de tejido renal normal (donantes cadáveres) se estudiaron, por inmunofluorescencia indirecta, para expresión de nefrina, podocina y α-actinina 4. Resultados: La expresión de estas proteínas fue lineal, homogénea, en las paredes capilares glomerulares del tejido renal normal y de pacientes con hematuria aislada. En proteinuria nefrótica y en algunos casos de proteinuria leve este aspecto normal estaba alterado y su expresión cambió de lineal a granular fina. En 22 casos (45%) de pacientes con proteinuria nefrótica y en 3 casos (30%) de pacientes con proteinuria subnefrótica hubo pérdida en la expresión de al menos una de estas proteínas (p = 0,49). Estas alteraciones se encontraron en las diferentes glomerulopatías que de forma más habitual causan síndrome nefrótico, aunque ninguna en particular fue significativamente más frecuente. Conclusiones: En la proteinuria nefrótica es muy frecuente la redistribución o la pérdida de proteínas asociadas al diafragma de filtración, lo que en muchos casos podría ser una consecuencia más que una causa de la proteinuria. Estas alteraciones pueden evidenciarse también en pacientes con proteinuria leve.
INTRODUCTION
Minimal change glomerular disease, membranous glomerulonephritis and focal segmental glomerulosclerosis (FSGS) are the most frequent glomerulopathies1-5 causing nephrotic syndrome. The latter is also the most common glomerular disease in Colombia, diagnosed by biopsy.6,7
The glomerular visceral epithelial cells are attached to the glomerular basal membrane by forming cytoplasmic extensions known as podocyte processes.8 Normally, the glomerular filtration passes between these podocyte processes through a specialised intercellular junction, unique to podocytes, known as slit filtration unit or slit diaphragm. In this study it will be called diaphragm filtration, as this best describes its structure and function. This structure is anchored to the cytoskeleton of the podocyte processes, and in this complex there are numerous structural proteins and messenger functions which are neither fully known or understood.9,10 So far, at least 15 proteins forming part of this complex have been characterised, and all of them are important in maintaining its structure and function: nephrin, podocin, NEPH1, 2 and 3, P-cadherin, CD2-associated protein (CD2AP), catenins, FAT 1 and 2, zonula occludens 1 (ZO-1), actin, alpha-actinin-4, densin and CRIM1.9,11-21 During the late 90s, with the discovery of several mutations in genes encoding these molecules, it was discovered that this is the main barrier for protein filtration.9,11,12,22
In response to many types of aggresion, podocytes suffer a morphological change called podocyte process ¿effacement¿ (or fusion) due to an architectural alteration of the cytoskeleton and intercellular union.8 By electron microscopy, this effacement occurs with retraction and free extension of podocyte cytoplasmic extensions. This process is reversible and is directly related to proteinuria in humans and experimental models.23
Molecular studies indicate that filtration diaphragm proteins form a repeated structure similar to a zipper with a periodicity of 40nm.9,24,25 The exact location and precise interactions between the diaphragm filtration proteins are not fully known. These proteins interact with the podocyte cytoskeleton and participate in intra- and intercellular signalling. It appears that the filtration diaphragm, besides being a filter depending on the size of molecules, also exerts its function in accordance with electric charge, and can repel proteins, preventing the diaphragm from becoming blocked.26
In case of alterations in some of the genes encoding these proteins, the whole complex can lose its normal structure and alter its function leading to nephrotic syndrome, usually congenital. However, in cases of acquired nephrotic syndrome, mediated or not by immune complexes and/or cellular immunity, podocyte alterations (podocyte process fusion or effacement) are described as a phenomenon secondary to nephrotic syndrome (or its cause).9,24 Therefore, it appears that the destruction and loss of the filtration diaphragm function and its complex anchoring proteins may be both the cause and consequence of nephrotic syndrome.
As FSGS is the most common glomerulopathy in Colombia, as diagnosed by biopsy,6,7 it is very important to look for molecular mechanisms related to its development to better understand its aetiology. Our goal was to look for an alteration in the expression of some of these proteins in Colombian nephrotic patients.
MATERIAL AND METHODS
Renal biopsies from patients with nephritic-range proteinuria (n = 40), in which fresh frozen tissue was made available after conventional immunofluorescence (IF), were selected to detect some of the proteins associated with filtration diaphragm: nephrin, C-terminus (C-ter), podocin, C-ter and α-actinin 4, C-ter. 11 cases of normal kidney tissue from kidney donor cadavers were used as normal controls, with microscopic study showing normal histology, IF negative for immunoglobulin deposits and/or complement fractions, and normal tests for renal function and urine cytochemistry. 10 cases of patients with non-nephrotic proteinuria and 3 cases of patients with isolated haematuria (without proteinuria or deterioration of renal function) were also included for comparison.
The samples came from patients aged between 1 and 71 years (median: 25), of whom 59.4% were men. The median age of patients with nephrotic proteinuria was 13.5 years (1-71), the median age of patients with non-nephrotic proteinuria was 33 years (9-67), the median age of patients with isolated haematuria was 11 years (6-52), and the median age of the cadaver donors with normal renal tissue was 48 years (20-60).
Inmunofluorescence
Renal biopsies were processed according to our systematic processing protocol based on international recommendations.27 Tissue fixed in formalin and embedded in paraffin was used for the study of conventional light microscopy. For direct IF (to detect IgA, IgG, IgM, C3, C1q, and kappa and lambda light chains), the samples were frozen immediately using gel suitable for frozen sections (Tissue-Tek, Nile, Inc, Eckhart, IN, USA), the necessary sections for diagnostic IF were made and the remaining tissue was further sectioned at 3μm thickness, and mounted on slides suitable for immunohistochemical techniques (Fisher Scientific, USA). These sections were fixed in acetone at room temperature for 10 minutes, then stored at -24ºC until used for the detection of proteins associated with the filtration diaphragm. In all cases, the presence of at least 5 glomeruli was confirmed before making the sections for this study.
For the detection of proteins associated with the filtration diaphragm, indirect IF was the technique used. All primary and secondary antibodies, the latter labelled with fluorescein isothiocyanate (FITC), are polyclonal and produced by the laboratory Santacruz Biotechnology Inc (USA): Nephrin, catalogue number sc32530; podocin, catalogue number sc22296; α -actinin 4, catalogue number sc7454; secondary antibody: anti-goat IgG from donkey and labelled with FITC: catalogue number: sc2024; normal donkey serum, catalogue number sc2044.
The IF process was as follows: the sections were thawed, washed with tris-phosphate buffer (TPB): two washes of 5 minutes, incubated with normal serum (to block non-specific binding) derived from the same species the secondary antibody originated from at 10% (1:10 dilution) for 20 minutes, washed with TPB for 5 minutes, incubated with primary antibody for 60 minutes, 1:20 dilution, washed three times for 5 minutes with TPB, incubated for 45 minutes with secondary antibody conjugated to FITC, dilution: 1:20, washed three times for 5 minutes with TPB, the histological slides were finally mounted with 90% glycerine and stored at 4° C in the dark until observation with the fluorescence microscope.
Interpretation
Immunostaining was evaluated on the walls of the glomerular capillaries and its intensity was subjectively evaluated by comparison with the controls of normal kidney tissue. The immunostaining pattern was recorded: linear or granular, and homogeneous or irregular (heterogeneous in different areas of the glomerulus). Static images were analyzed for all positive cases with Photoshop®, version 10, to determine the relationship of the immunostaining with the glomerular basal membrane.
Statistical analysis
The data were expressed as median (and minimummaximum values). To compare percentages, we used chisquare or Fisher tests, according to the number of cases and their values. Analysis was performed with SPSS® software, Chicago, IL, version 15.
RESULTS
Of the 40 patients with nephrotic range proteinuria, 37 had full nephrotic syndrome. Renal biopsy confirmed the diagnosis of FSGS in 15 of these cases, minimal change disease in 7, membranous glomerulonephritis (GN) in 8, IgA nephropathy in 2, postinfectious GN in 2, and 1 case of each of the following: type I membranoproliferative GN, dense deposit disease, diabetic nephropathy, amyloidosis, nephropathy associated with human immunodeficiency virus (HIV) and C1q nephropathy.
In the 10 cases of patients with non-nephrotic range proteinuria, the diagnosis was IgA nephropathy in 6 cases, and 1 each of FSGS, IgM nephropathy, hypertensive nephropathy and non-IgA mesangial proliferative GN. Three biopsies of patients with isolated haematuria whose renal biopsy diagnoses were: IgA nephropathy, thin basal membrane disease and non-IgA mesangial proliferative GN, were also included.
Expression of proteins associated with filtration diaphragm
Normal biopsies
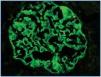
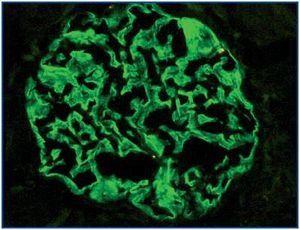
In the 11 normal kidneys, polyclonal antibodies against podocin, nephrin and α-actinin-4 show the same pattern of immunostaining: positive linear, homogeneous, diffuse in peripheral glomerular capillary walls (Figure 1). In some areas a location along the outer portion of the glomerular basal membrane was found. Henceforth, this immunostaining was considered as ¿normal¿.
Biopsies of patients with nephrotic proteinuria
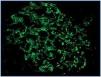
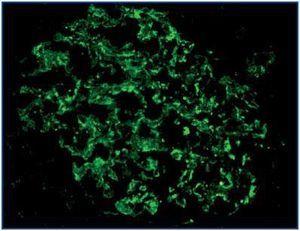
There were more cases with loss of expression of nephrin, podocin and α-actinin-4 in patients with nephrotic syndrome than in patients with subnephrotic proteinuria, although the difference was not statistically significant (p = 0.70, p = 0.57 and p = 0.66, respectively) (Table 1). The expression of these proteins was granular, with coarse or fine granules on the outside of the glomerular capillaries (Figure 2), and in most cases diffuse. This positivity was patchy and/or segmental in only a few cases, with hyaline lesions or segmental sclerosing. The expression of these proteins was not linear for any of the cases of nephrotic range proteinuria.
Biopsies of patients with non-nephrotic proteinuria
The expression was variable, with some cases of loss of expression of nephrin or α-actinin-4. There were no cases with loss of expression of podocin. The expression pattern (whether linear or granular) was variable, with some cases linear and others granular, with an appearance similar to that found in cases with nephrotic proteinuria (Table 1).
Biopsies of patients with isolated haematuria
In all 3 cases, the immunostaining for nephrin, podocin and α-actinin-4 was linear, similar to normal renal tissue. There were no cases with a loss of expression of any of these proteins.
In 2 cases, there was a simultaneous loss of expression of the three proteins studied; one of these cases corresponded to dense deposit disease and the other to HIV-associated nephropathy. In 5 cases there was a simultaneous loss of expression of two of these proteins, all in patients with nephrotic proteinuria; and in 14 cases there was a loss of expression of only one of the proteins studied, 11 patients with nephrotic syndrome and three patients non-nephrotic proteinuria. A total of 18 of the 40 cases (45%) of patients with nephrotic range proteinuria had a loss of expression of at least one of the proteins studied, and in 3 cases of non-nephrotic proteinuria (30%) there was a loss in expression of one of them (p = 0.49). None of the samples from patients with nephrotic proteinuria showed a simultaneous loss in the expression of several proteins.
No statistically significant differences were found in the expression of these proteins in different histological types of glomerular diseases. Although, it is striking that in minimal change glomerular disease, there were no cases of loss of expression of nephrin or podocin, and only 2 of 7 cases showed loss of expression of α-actinin-4 (Table 2).
The intensity of immunostaining varied somewhat between cases, but this variation was completely subjective as we did not have a system to quantify the intensity. In addition, this may vary according to the light exposure of each microscopic field, due to progressive loss of fluorescence with photoexposure. In segmental sclerosing lesions, it was common to find a loss of protein expression associated with filtration diaphragm. No expression was detected in extraglomerular sites for any of these proteins in any of the samples studied.
DISCUSSION
Genetic alterations in some of the proteins that are part of the filtration diaphragm have been associated with proteinuria or early-onset nephrotic syndrome. However, other non-genetic causes of nephrotic syndrome also produce these alterations.9,22,28 In this study we tried initially to find out if there were any common alterations in our patients with nephrotic syndrome, but we found no such alteration or characteristic associated with any of the glomerulopathies, including FSGS, which was the most common glomerular disease in our patients.
In our study, we found alterations in the expression of nephrin, podocin and the α-actinin-4 in cases of nephrotic range proteinuria, for whatever reason. This alteration was also evident in some cases of mild proteinuria (non-nephrotic), although less frequent. The loss of expression of some of these proteins is a common event in cases of nephrotic range proteinuria, but this does not indicate that there are mutations in the genes encoding them. The simultaneous loss of several of these proteins in some of our cases is indicative of a nongenetic alteration.
In normal kidneys, nephrin, podocin and α-actinin-4 appear together along the outside of the glomerular basal membrane. This normal distribution is lost in cases of nephrotic proteinuria, suggesting that the podocyte alteration found in these patients is directly related to the redistribution or loss of one or more of the proteins associated with filtration diaphragm. In cases of mutation of genes encoding either end of these proteins, there could be a loss in expression of this end, but the immunostaining of the other end may be maintained,29 or the mutated protein is still detected, but its location might change.30 This indicates that in our cases, although the immunostaining for one end of the nephrin, podocin or the α-actinin 4 is retained, this finding does not rule out the presence of mutations in genes that encode them. We tried to apply immunostaining to the other ends (Nter) of the nephrin, podocin and α-actinin-4, but the results with the antibodies used (the same manufacturer) were inadequate, and are, therefore, not included. Similarly, we tried to determine the alteration in different parts of the proteins CD2AP, TRPC6 and NEPH1 without achieving satisfactory results. The study of these other proteins would be very useful for studies of this kind, to be able to find out other alterations or to try to determine the frequency of simultaneous losses of other molecules.
The expression of nephrin, podocin and α-actinin-4 as nonlinear and granular indicates that these proteins have been redistributed in the podocyte cytoplasm and have not assembled as in the normal structure of glomeruli without disease. However, our results indicate that this may be a consequence of nephrotic syndrome of a different aetiology, and not necessarily a cause. It appears that podocytes somehow respond to proteinuria and alter the arrangement of these proteins. Consequently, these proteins represent a therapeutic target in proteinuria, as is suggested by modifying the proteinuria with the use of angiotensin converting enzyme inhibitors (ACEIs) or angiotensin II receptor antagonists (ARBs) in some patients who show changes in the expression of nephrin.20,21
These findings raise several important issues in the pathogenesis of proteinuria: (1) in severe proteinuria (nephrotic range), there is filtration diaphragm damage, which may be a cause and/or consequence of the proteinuria; (2) as well as the redistribution of these proteins, the loss of expression of some of them (or of some part in particular) is frequent, without necessarily implying any genetic alterations; (3) in non-nephrotic range proteinuria, although less common, alterations in the expression of proteins associated with filtration diaphragm are found, similar to those found in nephrotic syndrome; and (4) these alterations are related to proteinuria and are not found in cases of isolated haematuria.
In conclusion, in patients with nephrotic proteinuria, an alteration (redistribution or loss) of proteins associated with the diaphragm filtration is common. In many cases, this appears to be a consequence rather than a cause of the proteinuria. These alterations are also found, although less frequently, in patients with subnephrotic proteinuria.
Figure 1.
Figure 2.
Table 1. Expression of nephrin, podocin and α-actinin-4 in accordance with the clinical presentation
Table 2. Expression of nephrin, podocin and α-actinin-4 in the most frequent glomerulopathies