La timoglobulina forma parte del esquema de inmunosupresión en receptores de trasplante renal de alto riesgo inmunológico. Hemos comparado, en un estudio observacional y prospectivo, la incidencia de rechazo agudo, de infecciones oportunistas y de neoplasias, así como la supervivencia del injerto y del receptor, entre un grupo de 50 receptores de alto riesgo inmunológico con tratamiento de inducción que incluía timoglobulina, frente a un grupo de bajo riesgo cuyos 50 receptores recibieron injertos procedentes de los mismos donantes, en nuestro hospital en el período 2002-2006. El grupo de alto riesgo estaba formado por receptores hiperinmunizados (>50%), retrasplantes con pérdida de injerto previa inmunológica, reactividad en prueba cruzada, raza negra, o alta incompatiblidad HLA. La inmunosupresión consistió en administrar timoglobulina a dosis que mantuvieran un recuento de linfocitos T inferior a 10 μl, FK a partir del día 5, micofenolato mofetil y esteroides, y los pacientes recibían profilaxis frente al CMV con ganciclovir. El grupo de bajo riesgo incluía los pacientes sin estas características, a quienes se les realizaba la inmunosupresión con ciclosporina A, micofenolato mofetil y prednisona. Todos los receptores seronegativos con donantes seropositivos recibieron valganciclovir durante 100 días. Se descartaron aquellos pacientes en quienes se perdió el injerto por causas técnicas en el postoperatorio inmediato, junto con sus parejas. En todos los receptores se llevó a cabo un seguimiento mínimo de un año posterior al trasplante, con una mediana de 41,7 meses. Los dos grupos eran homogéneos en cuanto a edad y sexo del donante, edad del receptor e incompatibilidades HLA, pero el porcentaje de receptores varones era significativamente superior en el grupo control. El porcentaje de retrasplantes y de receptores hiperinmunizados fue significativamente superior en el grupo de alto riesgo, de acuerdo con los criterios de selección del grupo. La incidencia de rechazo agudo histológicamente probado fue superior en el grupo control (el 30 frente al 6%; p =0,003) y no se han producido diferencias significativas en cuanto a la incidencia de infecciones oportunistas ni de neoplasias; se ha diagnosticado un caso de leucemia aguda y un caso de enfermedad linfoproliferativa en el grupo de bajo riesgo. La supervivencia de los pacientes fue del 97,9% en ambos grupos al año y a los 3 años, mientras que la supervivencia del injerto fue del 89,8 y del 84,8% en el grupo de alto riesgo frente al 93,8 y al 90,4% en el grupo sin riesgo al año y a los 3 años (p = NS). En nuestra experiencia, la evolución de receptores de trasplante renal con alto riesgo inmunológico es similar a la del grupo de riesgo normal mientras se utilice una inmunosupresión lo suficientemente potente, que condicionó una incidencia de rechazo agudo significativamente menor en el grupo de alto riesgo.
INTRODUCTION
Thymoglobulin is a polyclonal agent made up of a vast variety of specific antibodies against several lymphocyte cell surface markers: CD2, CD3, CD4, CD8, CD11a, CD18, CD25, HLADR and HLA class I.1 Treatment with polyclonal antibodies induces significant T cell depletion, with remarkable inter-individual variation,2 which makes monitoring essential to prevent either acute rejection or excessive immunosuppression.3,4
Due to its potency, this drug is part of the immunosuppression regime for high immunological risk renal transplant recipients,4,5 while it is not included in the immunosuppression protocol used for low risk patients.6,7 Selection criteria for high risk group are set by clinical and immunological data for patients capable of developing a significant immunological response that would trigger a high rate of acute rejection and graft loss.4,5,8
We have evaluated the incidence of acute rejection, opportunistic infections, and non-dermatological malignancies, as well as graft and recipient survival, among a group of high immunological risk recipients with induction treatment that included thymoglobulin against a low risk group. Both group patients received grafts from the same donors.
MATERIAL AND METHODS
This is a prospective and observational study over recipients to renal graft transplanted in our hospital during the period between 2002 and 2006.
All grafts were from dead donors for 50 high immunological risk patients who were transplanted with their 50 low immunological risk counterpart patients.
The high risk group included patients with high rate of antibodies (> 50% antibodies against panel), recipients who had lost their first graft due to early rejection, cross match positive, black race, or HLA incompatibility according to immunology service centre criterion. Immunosuppression consisted in administering thymoglobulin to maintain T cell count at 10/μl, tacrolimus (FK506) after 5 days to reach serum concentration of 12-15ng/ml, mycophenolate mofetil (MMF) at 1,000mg/24 h and steroids (1mg/kg/24 h) in decreasing dosage. All recipients received ganciclovir prophylaxis for CMV for as long as they were given thymoglobulin. Ganciclovir dose was 1-1.25mg/kg and the mean by recipient was 5.5 dose/patient.
The low risk group included patients without the characteristics mentioned above; they were immunosuppressed with cyclosporine A (CYA), MMF and steroids (1mg/kg/24 h) in decreasing dosage. CYA dosing was set to reach 1,700ng/ml 2 hours after dose. MMF dosing was 2,000mg/24 h; it was reduced when intolerance or leukopenia appeared. All CMV seronegative recipients who received seropositive grafts received ganciclovir prophylaxis for 100 days. CMV infection was diagnosed when the patient developed clinical data of infection in presence of antigenaemia > 10 cells/2,000,000 leukocytes.
Acute rejection events were diagnosed following a histological study, and the affected patients received 500mg methylprednisolone IV daily during 3 days. When patient presented with resistance to the above treatment or acute rejection, Banff stage IIB or III, thymoglobulin was administered with the same monitoring pattern as in the prophylactic treatment.9
Recipients that lost their graft due to technical failure during immediate postoperative stage were excluded together with their counterparts. Minimum follow-up for all recipients was one year after transplant, with a mean of 41.7 months (percentile 25: 22.7; percentile 75: 57.2).
Statistical study
Description of patient baseline characteristics was performed through percentage for qualitative variables, and, for quantitative variables with normal distribution, mean standard deviation was used; while for those not following normal distribution mean percentiles were used. Chi square test was performed for qualitative variables, whereas for qualitative variables with parametric distribution Student¿s t-test was used. P < 0.05 was considered statistically significant.
Kaplan-Meier method was used to estimate survival function of survival times in both groups.
RESULTS
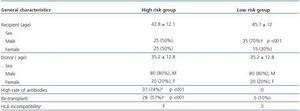
Both groups were similar with respect to gender and age, as they shared the same donors, recipient age and HLA incompatibilities, but the percentage of male recipients was significantly higher in the low risk group. The percentage of re-transplants and recipients with high rate of antibodies was significantly higher in the high risk group, according to the selection criteria of the study (table 1).
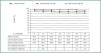
Regarding incidence by CMV infection and nondermatological malignancies during follow-up, no significant differences were observed (table 2).
The low risk group presented with two neoplasias, one acute leukaemia, with CYA, MMF, and steroids with no added risk factor; this required chemotherapy and immunosuppression switch to m-TOR (sirolimus) with good evolution; they also presented with a posttransplant lymphoproliferative disorder which evolved favourably but with graft loss when immunosuppressant was minimized and rituximab was administered. No episodes were observed in the high risk group (table 2).
The incidence of acute rejection was significantly higher (p < 0.03) in the low risk group (table 2) and 30% against 6% in the high risk group. There were episodes of acute rejection resistant to corticoids in the low risk group that were resolved with thymoglobulin in the three recipients.
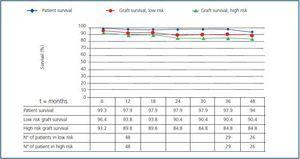
Recipient survival was 97.9% at 12 and 36 months in both groups, and the graft survival was 89.8% and 84.8% in the high risk group against 93.8% and 90.4% in the low risk group at 12 and 36 months (figure 1), with no significant differences observed.
Three patients died in the high risk group: two with functioning graft at 12 and 53 months, and the third at 42 months after chronically having lost his graft at month 4. Other 5 recipients lost their grafts, one of them due to acute rejection and the other at 4, 5, 17 and 24 months chronically; one of them due to non-compliance with treatment.
Two patients died in the low risk group, at 2 and 37 months due to abdominal sepsis and vascular pathology with functioning graft, respectively. Other 2 recipients lost their grafts chronically at 9 and 2 months, the last one when immunosuppression was minimised to treat diffuse lymphoproliferative disorder.
DISCUSSION
Inclusion criteria into the high immunological risk group were identical to those used in other studies and to those applied in clinical practice4,5,8 and permit identifying a group of patients with a theoretically high immunologic response. Some authors include delayed graft function when assessing a score to include recipient as high risk,4 however that is not our case.
In this series acute rejection rate was 6% in the group receiving thymoglobulin, a result that overlapped with other series published with the same characteristics regarding donor-recipient,10 but it was high in the low risk group recipients when compared against other series published with the same immunosuppression pattern.11 We must insist at this point in the fact that all acute rejections were diagnosed histologically, and no explanation has been found for disagreement regarding this information. This high rate of acute rejection, together with its lower incidence gathered in the literature with an immunosuppression pattern based on FK against cyclosporine,6,7 led us to change our immunosuppression protocol in patients with low immunological risk.
CMV infection incidence was similar in both groups. This fact can be explained by the use of universal ganciclovir prophylaxis in the high risk group and also due to the higher incidence of acute rejection in the low risk group, which was undoubtedly what conditioned an increase in immunosuppression for that group; all this confirms published data in that acute rejection is a risk factor for the development of infection by CMV.12 Valganciclovir prophylaxis in seronegative recipients who received seropositive grafts led to lower incidence of infection in this type of patients, which has already been described by other authors.13
Incidence of non-dermatological malignancies in both groups was low, with no incidence in the high risk group, which would be attributable to the number of patients in the series and to the fact that their follow-up was only for a medium term. It is striking that the only case of lymphoproliferative disorder after transplant occurred in the low immunological risk group, when it was to be expected amongst high risk patients, as these received thymoglobulin, which is considered a high risk drug fostering development of this disorder.14 In our experience the incidence of lymphoproliferative disorder is 1.3%,15 similar to that described in the literature,16 and no relation has been found in our series between this incidence and the administration of monoclonal or polyclonal antibodies.15 The therapeutic strategy to deal with posttransplant lymphoproliferative disorder consists in administering rituximab and changing immunosuppression to m-TOR, which is well documented in the literature,17 but which was not efficient with our patient.
Both patient and graft survival was similarly high in both groups; although it should be considered that early losses were excluded from the study. All leads to believe that the use of a potent immunosuppressant was decisive in following up graft survival in high risk group, and it is worth noting that it did not entail an increase in mortality of those recipients. Monitoring closely thymoglobulin doses by T-cell counts could have been an important factor, in low incidence of acute rejection as in the absence of side effects over immunosuppression. According to our experience, the evolution of high risk renal transplant recipients is similar to low risk patients if potent enough immunosuppression is used, which leads to significantly lower acute rejection incidence in the high risk group.
Table 1. General characteristics of high and low risk groups
Table 2. Incidence of episodes studied in high and low immunological risk groups
Figure 1.