Introducción y objetivos: Se ha descrito que el nivel de anticuerpos circulantes contra el receptor tipo M de la fosfolipasa A2 tiene correlación significativa con la actividad clínica de la enfermedad en la nefropatía membranosa idiopática (NMI). Sin embargo, la utilidad de la monitorización del título de anticuerpos como predictor de respuesta clínica tras el inicio del tratamiento no ha sido formalmente analizada. En el siguiente estudio se analiza el valor predictivo de la evolución del título de anticuerpos anti-PLA2R sobre la respuesta clínica en enfermos con NMI tratados con tacrolimus. Pacientes y métodos: 36 enfermos con síndrome nefrótico secundario a NMI, con criterios de indicación de tratamiento inmunosupresor, fueron tratados con tacrolimus en monoterapia. Se determinó el nivel de anticuerpos anti-PLA2R antes del tratamiento y a los 3, 6, 9 y 12 meses tras su inicio. Se analizó el valor predictivo de la pendiente de reducción en el título de anticuerpos y de la reducción absoluta y relativa en el título de anticuerpos a los 3 y 6 meses sobre el tiempo hasta la remisión y sobre la probabilidad de remisión a los 6, 9 y 12 meses. Resultados: La reducción relativa en el título de anticuerpos anti-PLA2R fue significativamente mayor en los enfermos que presentaron remisión y precedió a la respuesta clínica. No se apreció asociación entre el título de anticuerpos previo al tratamiento con el tiempo medio de respuesta o la respuesta a los 12 meses. La pendiente de reducción en el título de anticuerpos se asoció significativamente con el tiempo hasta la evidencia de remisión. La reducción relativa en el título de anticuerpos anti-PLA2R a los 3 meses tuvo una elevada sensibilidad y especificidad para predecir la respuesta a los 6 y 9 meses, pero no a los 12 meses, mientras que la reducción relativa en el título de anticuerpos a los 6 meses tuvo una elevada sensibilidad y especificidad para predecir la respuesta a los 12 meses. Conclusión: En enfermos con NMI asociada a anticuerpos anti-PLA2R, la monitorización del título de anticuerpos tras el inicio del tratamiento es útil para estimar el período de tiempo hasta la remisión y para predecir la probabilidad de remisión a los 12 meses.
Introduction and objectives: The level of circulating antibodies against M-type phospolipase A2 receptor has been reported as having a significant correlation with clinical activity in idiopathic membranous nephropathy. However, the usefulness of monitoring antibody titre as a predictor of clinical response following the onset of treatment has not been formally analysed. The predictive value of the evolution of anti-PLA2R antibody titre on the clinical response of idiopathic membranous nephropathy patients treated with tacrolimus is analysed in the following study. Patients and method: 36 patients with nephrotic syndrome secondary to idiopathic membranous nephropathy with immunosuppressive treatment indication criteria were treated with tacrolimus in monotherapy. The level of anti-PLA2R antibodies was determined before treatment and at 3, 6, 9 and 12 months after the onset of treatment. The study analysed the predictive value of the reduction in antibody titre and the relative and absolute reduction in antibody titre at 3 and 6 months over the period until remission and on the probability of remission at 6, 9 and 12 months. Results: The relative reduction in the anti-PLA2R antibody titre was significantly greater in those patients with remission and it preceded the clinical response. No association was observed between the antibody titre prior to treatment and the mean response time or the response at 12 months. Reduction in antibody titre is significantly associated with the time until signs of remission. Relative reduction in anti-PLA2R antibody titre at 3 months had a high sensitivity and specificity to predict the response at 6 and 9 months, but not at 12 months; however the relative reduction in the antibody titre at 6 months had a high sensitivity and specificity for predicting the response at 12 months. Conclusion: In patients with IMN associated with anti-PLA2R antibodies, the monitoring of antibody titre following the onset of treatment is useful for estimating the time period until remission and predicting the probability of remission at 12 months.
INTRODUCTION
Idiopathic membranous nephropathy (IMN) is an antibody-mediated disease caused by IgG and C3 deposits in the subepithelial space of the glomerular basement membrane1,2. There is currently agreement that patients with normal renal function who suffer from nephrotic syndrome for more than 6-12 months after diagnosis are candidates for receiving immunosuppressive therapy. The drugs whose efficacy has been proven in randomised clinical trials and which are considered the drugs of choice as the first line of treatment are alkylating agents combined with steroids and calcineurin inhibitors3,4. Both treatments have proven similar efficacy in inducing nephrotic syndrome remission and preserving renal function, but they have some limitations. Firstly, around 20% of patients may be resistant to one or both drugs3-13. Secondly, the available evidence suggests that after starting therapy, the probability of a response progressively increases with time, even beyond the period of exposure to the drug5-13, and there is currently no variable that allows us to predict whether the patient will respond or not to the treatment or the time at which the response will occur. Recently, the M-type phospholipase A2 receptor has been identified as one of the target antigens of the autoimmune response in IMN patients14-16 and it has been reported that circulating antibodies against the latter (anti-PLA2R), present in approximately 70%-75% of patients, have a significant correlation with the clinical activity of the disease17,18. As such, the evolution the anti-PLA2R antibody titre after the start of treatment could be useful for predicting the response. Some evidence indicates that after treatment with rituximab the reduction in the antibody titre precedes the remission of proteinuria19. However, the usefulness of monitoring the antibody titre as a predictor of clinical response has not been formally analysed and there are no data on the evolution of the anti-PLA2R antibody titre in patients treated with tacrolimus.
In this study, repeated measurements of the anti-PLA2R antibody titre were carried out before and during the 12 months following the start of treatment in patients with IMN who received tacrolimus, with the objective of analysing the predictive value of the anti-PLA2R antibody titre evolution on the clinical response.
PATIENTS AND METHOD
We included a total of 36 patients who fulfilled the following criteria: 1. Age >18 years old. 2. Nephrotic syndrome caused by IMN confirmed by renal biopsy. 3. Exclusion of secondary aetiologies. 4. Anti-PLA2R antibody titres >20RU/ml at the time of diagnosis. 5. Immunosuppressive therapy criteria due to persistence of the nephrotic syndrome after 6 months of symptomatic treatment with angiotensin receptor blockers, statins, diuretics and a low-sodium diet, in accordance with the treatment guidelines3,4. 6. Normal renal function, as defined by a CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) value >60ml/min/1.73m2.
The renal biopsies were stained with haematoxylin and eosin, PAS, methenamine silver and Masson's trichrome stainings for the morphological analysis and we carried out immunofluorescence studies with antibodies against IgA, IgG, IgM, C3, fibrinogen and light chains. The diagnosis of IMN was carried out in the presence of a compatible morphological pattern, associated with evidence of subepithelial IgG and C3 deposits in the immunofluorescence.
At the time of diagnosis, patients received treatment with angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers, a low-sodium diet and treatment for dyslipidaemia with statins. After a 6-month observation period and after observing no spontaneous remission, all patients received monotherapy with tacrolimus at an initial dose of 0.06mg/kg/day, which was subsequently adjusted to maintain target levels of 7ng/ml-9ng/ml after 12 hours. No patient had previously received immunosuppressive therapy. Treatment with tacrolimus was maintained for three months after evidence of remission or a maximum of 12 months in cases in which patients had not shown remission at the end of this period. After evidence of total or partial remission, the tacrolimus dose was reduced at a rate of 30% per month until total suppression or recurrence.
Per protocol, all patients were tested monthly until there was evidence of remission, and if there was remission, they were tested every 2-3 months until suppression of treatment, evidence of recurrence or the absence of a response after 12 months. In all patients included, serum samples were extracted before and after the start of treatment every three months until the end of the first year. These samples were used to determine the anti-PLA2R antibody titre by ELISA (Euroimmun, Lübeck, Germany; linearity: 6-1500RU/ml; lower limit of detection 0.6RU/ml).
The outcome variables were the probability of obtaining total or partial remission in the course of the 12 months following the start of treatment, the time period between the start of treatment and evidence of remission, the evolution of the antibody titre during the observation period and the percentage of patients who had a negative antibody titre at the end of the 12-month observation period.
Definitions:
- Complete remission: proteinuria <0.3g/day, albumin >3.5g/dl and glomerular filtration rate >60ml/min/1.73m2.
- Partial remission: >50% reduction in baseline proteinuria, with the last test showing <3.5g/day and with a glomerular filtration rate >60ml/min/1.73m2.
- No response: absence of complete or partial remission 12 months after the start of treatment.
- Negative antibody titre: anti-PLA2R antibody titre <20RU/ml.
This study followed the parameters of the Declaration of Helsinki. All patients gave their written informed consent and the study was approved by the hospital’s bioethics committee.
Statistical analysis
The results are expressed as a mean and standard deviation for the variables with normal distribution and as a median and quartiles for variables whose distribution is not normal. The differences in proportions were analysed using the χ2 test or Fisher’s exact test. The analysis of the antibody titre evolution over time was carried out using analysis of variance for repeated measurements. In order to study the predictive value of the reduction in the antibody titre on the response, we calculated the absolute and relative reduction of the antibody titre after 3 and 6 months in relation to the baseline value, as well as the reduction slope in the antibody titre during the follow-up period, expressing it in RU/ml/month. We carried out a univariate analysis to identify the variables associated with the probability of remission during the 12-month observation period through the Kaplan-Meier method, using the log-rank test for comparison between groups. The relationship between the reduction slope in the antibody titre and time until evidence of remission was calculated using a simple linear regression. Using ROC curves, we analysed the anti-PLA2R antibody titre reduction value after 3 and 6 months that had the greatest sensitivity and specificity for identifying patients in remission after 3, 6, 9 and 12 months. Differences were considered to be statistically significant when p was <.05. The version 20.0 SPSS statistical software was used.
RESULTS
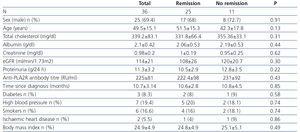
Table 1 summarises the baseline clinical and biochemical characteristics of the total number of patients included in the study in terms of their response to treatment after 12 months. No significant variables were observed in any of the variables analysed between those patients who responded and those who did not.
Over the 12 months of follow-up (Figure 1), the probability of remission increased progressively over time, with a 40% probability after 6 months, 60% after 9 months and 69.4% (25/36 patients) after 12 months. Of the total number of patients in remission, 9 (25%) showed complete remission and 16 (75%) showed partial remission. The median time between the introduction of treatment and evidence of remission was 8.5 months (interquartile range: 5.5-9.6 months). The percentage of patients who entered total or partial remission without treatment with tacrolimus after 12 months was 28% (7/25). The mean tacrolimus dose was 0.05±0.017mg/kg/day and the mean level was 7.9±1.9ng/ml without significant differences being observed between those who responded and those who did not. During the observation period, there was no recurrence in any patient when tacrolimus therapy was discontinued.
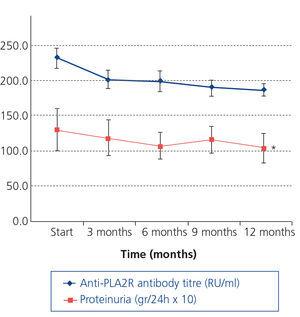
Figure 2 displays the evolution of the antibody titre according to the response to treatment in the total group of 36 patients studied. In relation to the baseline value, in the group of patients, the relative reduction in the antibody titre at the end of the 12-month observation period was 61.3±37% (p:.004). There were no differences in the baseline antibody levels between patients who entered remission and those who did not. Following treatment, the antibody titre after 3, 6, 9 and 12 months was significantly lower in those who showed total or partial remission (F: 25.9, p:.000) than in those who did not respond to treatment. The mean anti-PLA2R antibody titre at the time of remission was 17.4±8.3RU/ml. Twenty of the 25 (80%) patients who entered remission had an anti-PLA2R antibody titre <20RU/ml at the time of remission. Figure 3 displays the evolution of the anti-PLA2R antibody titre and proteinuria in patients who did not respond to treatment. We can observe a statistically significant reduction in the antibody titre between the baseline level and the third month (18.5±4.8%, p:.037) and an accumulated 21.4±13.6% reduction in the antibody titre after 12 months. The reduction slope in the antibody during the 12-month follow-up in patients who did not respond to treatment was 7.53±5.50RU/ml/month. Over the course of the follow-up period, we did not observe significant changes in urine protein excretion.
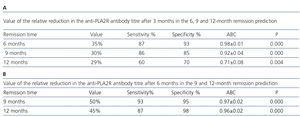
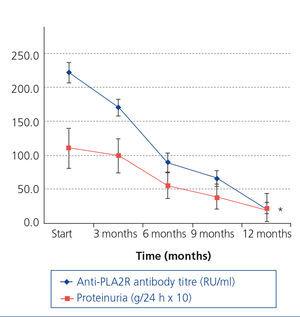
Figure 4 displays the evolution of the anti-PLA2R antibody titre and proteinuria in patients who showed remission. The relative reduction in the antibody titre preceded the decrease in proteinuria and it was statistically significant from the third month. The relative reduction in the antibody titre was 35±18.4% after 3 months, 60±16.4% after 6 months, 70±12% after 9 months and 78.9±14.2% after 12 months. The reduction slope in the antibody titre during the observation period in patients who responded to treatment was -21.5±3.78RU/ml/month. Using a simple regression analysis, we observed a statistically significant relationship between the antibody titre reduction rate and the time until remission (Table 2). Table 3A summarises the value of relative reduction in the antibody titre after three months on the prediction of response to treatment after 6, 9 and 12 months. Table 3B summarises the value of the relative reduction in the antibody titre after 6 months on the prediction of the response to treatment after 9 and 12 months. It can be observed that the relative reduction in the antibody titre after 3 months has increased sensitivity and specificity for predicting the response after 6 and 9 months but not after 12 months, while the relative reduction in the antibody titre after 6 months has an increased sensitivity and specificity for predicting the response after 9 and 12 months.
DISCUSSION
In this study, we carried out repeated measurements of the anti-PLA2R antibody titre before and during the 12 months following the start of treatment in patients with IMN who received tacrolimus as the first line of treatment, with the objective of analysing the predictive value of the evolution of the anti-PLA2R antibody titre on the clinical response. The patient group included a non-selected sample of patients with IMN associated with anti-PLA2R antibodies, who were treated by following the same protocol, after an extended period of symptomatic treatment starting at diagnosis in accordance with recommendations of different guidelines3,4. The observation period was 12 months, which is the maximum period in which monotherapy with tacrolimus was maintained if no response was observed. Both the probability of remission observed over time and the percentage of patients in remission after 12 months observed in our patients were very similar to those reported in previous studies with calcineurin inhibitors11-13. For these reasons, the results observed in our patients could be applied to other patient groups with IMN who receive tacrolimus.
The results of our study provide the following data of clinical interest. Firstly, similarly to the data reported after treatment with rituximab19,20, our results indicate that in patients treated with tacrolimus, the reduction in the anti-PLA2R antibody titre after the start of treatment precedes the remission of proteinuria and is related to the clinical response. The evidence of a progressive reduction in the antibody titre was highly predictive of remission, while persistence of high titres was associated with the persistence of nephrotic syndrome, with statistically significant differences in the antibody titre between patients with and without a response, which were evident from the third month and they were maintained throughout the whole observation period. Furthermore, in patients who responded to treatment, the antibody level reduction rate was associated with the speed of the clinical response. Both data indicate that monitoring the anti-PLA2R antibody titre after the start of treatment is useful in clinical practice and it may have two practical implications. The first is that the antibody titre reduction rate after the start of treatment may be used as an estimator of the response time. The second is that the relative reduction in the antibody titre after 3 and 6 months may serve as a guide for predicting the response probability. Given that the available evidence indicates that the response to treatment with tacrolimus increases progressively with the drug exposure time13 and that none of the baseline clinical or biochemical characteristics allow the probability of response to treatment to be calculated, in patients who show late responses, a relative reduction value in the antibody titre equal to or greater than 50% after 6 months may be a useful criterion when making the decision to maintain immunosuppressive therapy, while the persistence of reductions lower than this figure after 6 and 9 months has to suggest a high probability of resistance to treatment.
IMN is an antibody-mediated glomerulopathy. In approximately 70% of cases, these antibodies are directed against the M-type phospholipase A2 receptor. In 30% of cases, the antibodies are not identified either in their circulating form or deposited in the glomerular basement membrane. The identity of antibodies in the immune deposits responsible for glomerular damage and dysfunction in these cases is at present uncertain. Calcineurin inhibitors (cyclosporine and tacrolimus) induce downregulation of a series of cytokines synthesised by T helper cells (Th1 and Th2) and antigen-presenting cells, particularly interleukin 2, which amongst other functions is involved in the activation of B lymphocytes and the subsequent production of antibodies21. This would be the key mechanism through which calcineurin inhibitor therapy induces the reduction observed in the anti-PLA2R titres and its correlation with remission.
In summary, our data indicate that in patients with IMN, the reduction slope in the anti-PLA2R antibody titre is significantly associated with the time until evidence of remission. Furthermore, the relative reduction in the anti-PLA2R antibody titre after 3 months is very sensitive and specific for predicting the response after 6 and 9 months, but not after 12 months, while the relative reduction in the antibody titre after 6 months has a high sensitivity and specificity for predicting the response after 9 and 12 months. These data may be useful in the clinical follow-up of patients after the start of therapy and they may be particularly important in cases in which, as has been recently proposed22,23, we consider the anti-PLA2R antibody titre as the base for treatment decisions.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Baseline clinical and biochemical characteristics and therapeutic response
Table 2. Relationship between the antibody titre reduction slope and the time until remission in patients who responded to the treatment
Table 3. Value of the relative reduction in the level of anti-PLA2R antibodies in relation to the baseline level in the 6, 9 and 12 month remission prediction
Figure 1. Probability of remission and time in the sample studied.
Figure 2. Anti-PLA2R antibody titre and remission in the sample studied.
Figure 3. Evolution of proteinuria and the anti-PLA2R antibody titre in patients without remission in the observation period.
Figure 4. Evolution of proteinuria and the anti-PLA2R antibody titre in patients with remission in the observation period.