Antecedentes y objetivos: El síndrome nefrótico es una enfermedad crónica especialmente común en la infancia y la adolescencia. Las especies reactivas del oxígeno (ERO) y los radicales libres desempeñan un papel importante en la patogénesis del síndrome nefrótico. El objetivo de este estudio es evaluar los efectos de la genisteína (principal isoflavona de la soja) y la proteína de soja en el estado antioxidante renal de ratas nefróticas. Métodos: Este estudio se llevó a cabo durante 8 semanas con 40 ratas Sprague-Dawley machos adultas, que fueron divididas en cuatro grupos de 10. Cada uno de los grupos de estudio incluía: 1 control, 2 con síndrome nefrótico, 3 con síndrome nefrótico más una dieta a base de proteína de soja y 4 con síndrome nefrótico más una dieta a base de proteína de soja más genisteína. Se midieron tanto los niveles de proteína como de creatinina en orina. Tras la homogenización del tejido renal, se calcularon mediante espectrofotometría la capacidad antioxidante total (CAT), la actividad de la enzima catalasa, la concentración de malondialdehidos (MDA) y las proteínas carboniladas. El examen patológico de los riñones se realizó con el microscopio óptico. Además, se evaluó la viabilidad celular con un ensayo de MTT de la línea celular de fibrosarcoma WEHI-164. También se evaluó la actividad de la enzima MMP2 con distintas concentraciones de genisteína. Resultados: La capacidad antioxidante total aumentó significativamente en las ratas que tenían una dieta de genisteína, al igual que la actividad de la catalasa en aquellas con una dieta de soja y genisteína. En cambio, los grupos carbonilo de las proteínas y los niveles de MDA fueron significativamente inferiores en los animales con una dieta de soja y de genisteína. El examen patológico reveló una mejora significativa en los grupos con dietas de soja y de genisteína. Asimismo, la genisteína disminuyó la proliferación de la línea celular de fibrosarcoma WEHI-164. Conclusión: Parece ser que la proteína de soja reduce los daños renales causados por el síndrome nefrótico. La adición de genisteína a la proteína de soja produce mejoras en el estado antioxidante del tejido renal. La genisteína disminuye la proliferación celular.
Background and objectives: Nephrotic syndrome is a chronic disease especially common in the childhood and adolescence. Reactive oxygen species (ROS) and free radicals have significant role in the pathogenesis of nephrotic syndrome. The aim of this study was to evaluate the effect of soy protein and genistein (main isoflavone of soybean) on renal antioxidant status of nephrotic rats. Methods: This study was done for 8 weeks on 40 adult male Sprague-Dawley rats divided into four groups of 10 rats each. Study groups included: 1-Control, 2-Nephrotic syndrome, 3-Nephrotic syndrome+soy protein diet and 4-Nephrotic syndrome+soy protein diet+genistein. Urine protein and urine creatinine were measured. After homogenization of kidney, total antioxidant capacities (TAC), activities of catalase enzyme, the concentration of malondialdehydes (MDA) and carbolynated proteins were determined spectrophotometrically. Pathological examination was done on kidneys with light microscope. Cell viability was evaluated with MTT assay on WEHI-164 fibro sarcoma cell line. The MMP2 enzyme activity was evaluated in different concentrations of genistein. Results: Total antioxidant capacity was significantly increased in soy genistein. Catalase activity was significantly increased in soy and soy genistein groups. Protein carbonyl and MDA were significantly lower in soy and soy genistein groups. The scores of pathological examination showed significant improvement in soy and soy genistein groups. Genistein decreased the proliferation of the WEHI-164 fibrosarcoma cell line. Conclusion: It seems that soy protein decreases kidney damages in nephrotic syndrome. Adding genistein to soy protein causes improvements in antioxidant status of kidney tissue. Genistein decreases proliferation of cell.
INTRODUCTION
Nephrotic syndrome is a chronic disease mostly in the childhood and adolescence.1 It is due to alterations in the penetrability of the glomerular barrier, resulting from the activities of proteases and a decline in the synthesis of the proteoglycans.2 These malfunctions result in the emergence of a series of symptoms including severe proteinuria, hypoalbuminemia, hyperlipidemia and edema.3 Decreases of the glomerular blood flow and level of glomerular filtration rate (GFR) result from vasoconstrictor bioactive lipids (prostaglandin, thromboxane, platelet-activating factors (PAF)) and inhibition of nitric oxide activity.2,3 There are several evidences available concerning the roles of reactive oxygen species (ROS) and free radicals in the pathogenesis of nephrotic syndrome.3,4 The sources of ROS are the electron transport chain, oxidant enzymes, phagocytosis and auto-oxidation from epinephrine.2,4 Free radicals have great contribution in the damages resulted in DNA, proteins, carbohydrates and lipids destruction.5 ROS results in lipid per-oxidation in the cellular and organelles membranes. It leads to loss of cell’s structure and capacity to transmit and produce energy, especially in the proximal tubules. Meanwhile, ROS are produced in nephritis by the immunity system, the infiltrated blood cells (polymorphonuclear, leukocytes, and monocytes), podocytes and mesangial matrix cells.2 There are various antioxidant defensive mechanisms for protecting the cells against the harmful effects of ROS and free radicals. Some of these defensive mechanisms are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and glutathione reductase (GR).6 In nephrotic syndrome, the balance between the oxidant and antioxidant is lost.3 Some studies showed the decreasing trend in the activities of the antioxidant enzymes and vitamins C, E, beta-carotene and total antioxidant capacity, while the MDA level increased.7 In order to generate the nephrotic syndrome experimental model in the rats, adriamycin and/or puromycin amino nucleoside may be used, while their metabolisms resulted cause formation of reactive oxygen species (ROS), followed by appearance of severe proteinuria and decreased kidney performance.8,9 The protective effects of some of compounds against the oxidative damages are associated to their capacity to increase the antioxidant enzymes expression. Lack of foodstuffs containing antioxidant or suppression of antioxidant enzymes worsens the kidney damages resulting from free radicals.10 Soybean with the same protein quality as animal proteins is considered the richest source of isoflavones. Its isoflavones are Genistein, Daidzein and Glystein. They have estrogenic effects, therefore they are called phytoestrogens.11 It has been observed that foodstuffs containing soybean may have useful effects on the cardiovascular and renal diseases.12 Scientists believe that isoflavones, by their antioxidant nature, neutralize free radicals and decrease inflammatory reactions.13 There have been addressed several mechanisms concerning the effect of soybean on the renal diseases; however, they have attributed all the effect of soybean on kidney to its Genistein.10
In various studies, the roles of Genistein, as the major soybean isoflavone, have been studied in a variety of cellular mechanisms, e.g. 1) Apoptosis induction; 2) Cellular differentiation; 3) Prevention of cellular reproduction; 4) Controlling cell cycle progression; 5) Antioxidant effect; 6) Repetitive effectiveness of the treatment-resistant anticancer drugs; 7) Angiogenesis controlling; 8) Inhibiting osteoclast; 9) Controlling cellular immunity activity and its extension; 10) Mast-cell sustainability and decreased inflammation.14 The purpose of this study is to examine the effects of Genistein as an isoflavone, together with the soybean protein, on the glomerulosclerosis induced by adriamycin, and also studying the potential cytotoxicity and preventive effects of Genistein on cellular proliferation and activity of matrix metalloproteinase (MMPs) on the WEHI-164 fibrosarcoma cell lines.
MATERIALS AND METHODS
This study was done on 40 adult male Sprague-Dawley rats. 12-15 weeks old rats weighing 300±50g were obtained from Iranian Pasteur Institute and housed under standard condition of the animal room (temperature 25±3 centigrade degrees, humidity 50%, 12-hour light and 12-hour darkness) with free access to food and water. Then, they were randomly divided into four groups, of 10 rats each. The study protocol was approved by ethic committee of Tehran University of Medical Sciences (TUMS) which conforms to the provisions of the Declaration of Helsinki.
Study groups included
Diets
In order to provide soy protein diet, AIN-93M was followed and casein protein was replaced with the same amount of soy protein (soy protein as 14.1% of total energy). Due to the lack of sufficient amount of methionine, it was added to soy diet prohibiting methionine deficiency (Table 1).15
Disease induction and intervention protocol
All animals obeyed their diet for 2 weeks (chow diet for control and NS groups and soy diet for soy and soy-genistein groups). Then, the induction of nephrotic syndrome was done by the intravenous injection of single dose of 8mg/kg Adriamycin (Adriablastina, Farmitalia, Milan, Italy) in all groups except control.8 The study continued for 6 other weeks with each group receiving its especial diet. NS+S+G group also gavaged with 40mg/kg/day genistein diluted in CMC, while NS+S rats received CMC alone. At the end, the kidneys were brought out, washed with PBS and the right one was transferred to formalin for pathology examination and the left to sterile test tubes. The left kidneys were kept in -80.
Determination of the Kidney Performance Status
Urine collections were done for each group. Urine protein was measured colorimetrically by Pyrogallol-red kit (cat no. 10-545 ZiestChem Diagnostics, Tehran Iran). Urine creatinine was measured based on the Jaffe method (Pars Azmoon Co. Tehran, Iran).
Kidney Tissue Homogenization
The frozen left kidneys were homogenized in buffer (PH=7/4 0.05% NaN3, 25mM Tris-Hcl, 2mM PMSF, 2mM EDTA) by sonication. The sonication process was done in five minutes with six cycles. After being centrifuged, the supernatants were separated and transferred to -80˚C.
Evaluation of the oxidative stress parameters
Total antioxidant capacities (TAC) of the kidney tissues were measured in supernatants with 2-2- Azino- bis- (3- Ethyl benzthiazoline-6-Sulfonic acid) (ABTS) method.16 The activities of catalase enzyme in supernatans were determined by the HegoAebi’s method.17 the concentration of malondialdehydes in supernatants were measured spectrophotometrically with the thiobarbituric acid.18 The carbolynated proteins in supernatants were determined spectrophotometrically based on the reaction of carbonyl group with 2,4 dinitrophenylhydrazine.19
Kidney histopathology
The severity and extensiveness of the glomerular damages were assessed by pathological examination on tissue slices with optical microscope. The severity of glomerulosclerosis was evaluated by 8 parameters including hypercellularity, lobular pattern, polymorphonuclear (PMN) infiltration, atrophy of lumen, degenerative necrotic changes, hyaline cast in lumens, interstitial leukocyte infiltration and fibrosis. These parameters were categorized into scales ranging from 0 to 3 (0=negative, 1=mild, 2=average, 3=severe).
Cytotoxicity Studies
Cell Culture
The WEHI-164 fibro sarcoma cell line obtained from Iran Pasteur Institute was cultured in the media containing L-Glutamine, 10% FBS, RPMI and 100Unit/mL Penicillin/Streptomycin and incubated in 37ºC, saturated moisture and 5% CO2 pressure.
Proliferation assay
Cell viability was evaluated with MTT assay. MTT colorimetric test is based on the activity of the mitochondria dehydrogenase enzyme of the living cells in reduction of the MTT salt to the formazan non-solvable crystals, which could not pass through the cells’ membrane. The concentration of formazan is proportional to the number of viable cells.20 Some of the WEHI-164 fibro sarcoma cell line was cultured in 96-cell plates with 8x103 cells in a well (incubated for 4 hours under 37ºC). Genistein suspended in RPMI was added to each well in 0, 1, 5, 10, 20, 40 & 80μg/well, concentrations. The plate was then incubated for 48 hours in incubator (37ºC). The respective supernatant was extracted. Two hundreds microlitres of the MTT solution with 0.5mg/mL concentration in phenol red-free media was added to each well and incubated for another 2-4 hours. The supernatants were extracted and 200μL of DMSO was added to each plate as solvent of the formazan crystals (purple precipitation). The plate light absorption was read with 570nm wavelength and 630nm reference wavelength by ELISA reader machine.
Gelatinase Zymography
The mentioned-above cell line was cultured in 24-cell plates with 4x104 cells in a well. They were incubated for 4 hours allowing the cells to adhere to the plate and reaching to proper density. Then various densities of Genistein (0-400μg/mL) was added to each well and incubated for 48 hours under 37ºC, saturated moisture and 5% Co2 pressure. Separating the supernatants, they were centrifuged to remove cellular debris and kept under -20ºC until performing Zymoanalysis.
The MMP2 enzyme activity was evaluated based on the method described elsewhere.21 In brief, Genistein-containing supernatants were aliquoted and electrophorized with the SDS-PAGE gel containing 0.5mg/mL of A gelatin (Merk Germany) and the tris-borate as buffer for 3 hours (100V). Then, the gel was washed with X-100 triton two times to extract SDS and incubated for 24 hours in Tris-Hcl gelatinase activation buffer, PH=7.4 containing 10mM Cacl2. After that, the gel was immersed in Coomissie Brillient Blue-G-250 1% (1% in 25% methanol and 10% acetic acid solved in water) for two hours. MMP-2 activity resulted in gel proteolysis and generation of brilliant bands on the blue background. The activity of MMP-2 was measured by using the Alpha Ease FC densitometry (Alpha Innotech- Mimi, USA) comparing width and density of the bands appeared on the gel.
Statistical Analysis
The data has been shown in Mean±SD. Statistical package for the social sciences (SPSS, version 20, Chicago, IL) was used for statistical analysis. Parametric data analysis was performed by analysis of variance (ANOVA) and Post hoc test by Tukey-HSD. While the non-parametric data were analyzed by Mannwhitney & Kruskalwallis Tests. The data was considered meaningful with P value less than 0.05.
RESULTS
The urine protein to creatinine ratio was not significantly different among four groups at the beginning of the study (P=0.16) (Table 2). At the end of the study, the urine protein to creatinine ratio showed statistically significant difference among four groups (P<0.001). The normal group had the urine protein to creatinine ratio significantly different from other three groups (P<0.001) (Tukey HSD test). Disease induced groups including patients, soy and soy genistein showed differences in the urine protein to creatinine ratio but it was not significant. The urine protein to creatinine ratio was obviously less than the patient group in soy and soy genistein groups. The urine protein to creatinine ratio in soy genistein group was even lesser than it in soy group.
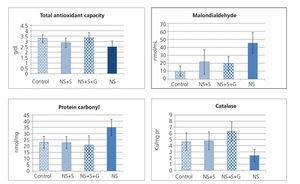
Figure 1 shows malondialdehyde, protein carbonyl, total antioxidant capacity (TAC) and catalase enzyme activity in the kidney tissue of animals. TAC was significantly different among four groups (P=0.008). In post hoc comparison, tukey HSD test showed significant difference between nephrotic rats vs. control (P=0.036) and soy genistein (P=0.011). The groups were significantly different in tissue catalase activity (P<0.001). In multiple comparisons, the NS group showed significant difference with control (P = 0.045), soy (P=0.014), and soy genistein (P<0.001). The amount of protein carbonylated in kidney tissue was different between groups (P=0.001). The protein carbonylated in kidney tissue had significant difference between NS and control (P=0.01), NS and soy (P=0.003), NS and soy-genistein (P=0.001). Malondialdehyde in kidney tissue was significantly different among groups (P<0.001) (Figure 1). The amount of malondialdehyde in tissue was significantly different between NS and control (P<0.001), NS and soy (P=0.004), NS and soy-genistein (P=0.002).
The effect of genistein on cellular proliferation
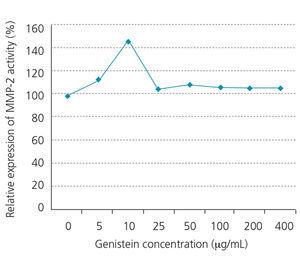
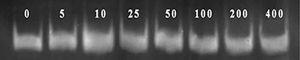
As Figure 2 shows, the increase in the genistein concentration accompanies with decrease in proliferation of the WEHI-164 fibrosarcoma cell line, while the decreased growth has been quite obvious under 5-80μg/well density. In addition, we observed obvious increase in MMP-2 activities at 10µg/mL concentration of genistein (Figure 3 and Figure 4). This effect was not shown in higher concentrations.
Histopathological Findings
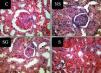
Table 3 shows the histopathological findings in different parameters. We compared the parameters of only three groups (NS, NS+S and NS+S+G). Hypercellularity parameter was significantly different among three groups (P<0.001) (Figure 5). The score of hypercellularity in NS group was significantly higher than that of soy and soy-genistein groups (P=0.022 and P=0.01, respectively). The score of hypercellularity was also significantly lower in soy soy-genistein group in comparison to soy group (P<0.001). The score of lobular pattern was significantly different among three groups (P<0.001). Scores were significantly lower in soy-genistein group in comparison to soy (P<0.001) and NS groups (P=0.001). The score of PMN infiltration was significantly different among groups (P<0.001). Score of PMN infiltration was significantly lower in soy-genistein group in comparison to soy (P<0.001) and NS groups (P=0.001). The score for degenerative-necrotic changes was significantly different among three groups (P=0.001). Its score was significantly lower in soy-genistein group in comparison to soy (P=0.012) and NS groups (P=0.001). The soy and NS groups were significantly different based on the score of degenerative-necrotic changes (P=0.022). The score for tubular atrophy was significantly different among three groups (P<0.001). Its score was significantly lower in soy-genistein group in comparison to NS group (P=0.001). The soy and NS groups were significantly different based on the score of tubular atrophy (P=0.002). The score for hyaline cast was significantly different among three groups (P<0.001). Its score was significantly lower in soy-genistein group in comparison to soy (P<0.001) and NS groups (P=0.001). The soy and NS groups were significantly different based on the score of hyaline cast (P=0.016). The score for leukocytes infiltration in interstitial was significantly different among three groups (P=0.001). Its score was significantly lower in soy-genistein group in comparison to soy (P=0.021) and NS groups (P=0.001). The soy and NS groups were significantly different based on the score of leukocytes infiltration in interstitial (P=0.007). The score for interstitial fibrosis was significantly different among three groups (P<0.001). Its score was significantly higher in NS group in comparison to soy (P<0.001) and soy-genistein groups (P=0.001).
DISCUSSION
This study showed adding genistein to soy protein can decrease histological deteriorations in nephrotic syndrome. Soy protein, with or without genistein, decreased tissue malondialdehyde in nephrotic rats. Soy protein increased catalase activity and diminished carbonylated protein of kidney tissue in nephrotic syndrome. These findings support the beneficial effects of soy protein in nephrotic status. The beneficial effects of soy could be associated to its copious isoflavone, genistein. Genistein makes improvements in nephrotic syndrome through its antioxidant effects.10,11 Some findings support the pivotal role of oxidative stress in nephrotic syndrome. The imbalance between oxidant and antioxidant factors causes the glomerular damage in renal diseases.3 The activities of antioxidant enzymes decrease in nephrotic syndrome. Hence, the peroxidation products increase in kidney diseases. Malodialdehyde and carbonylated proteins are produced through peroxidation processes.
Previous studies showed that diets containing soy could decrease proteinuria in comparison to casein protein diets.10,13 In the process of nephrotic syndrome, serum albumin is lost through kidneys. Serum albumin functions as a major antioxidant in serum, hence albumin loss causes oxidative damage. In the case of albumin loss, MDA as a product of oxidative damage increases.3 Isoflavones mainly genistein restrict inflammation with their antioxidative, anti inflammatory and anti necrotic characteristics.13 The anti inflammatory effect of genistein is through inhibition of cyclooxigenase expression and myeloperoxidase activity.22 Genistein decreases lipid peroxidation and serum lipids.23 Oxidative stress induces NF-kB and inflammatory cytokines expressions.24 Isoflavones reacts with free radicals and neutralizes their effects.13 It seems isoflavones decrease renal damage through reacting with hydrochloric acid and peroxynitrate.10 More studies are needed to analyze the angiotensin-II receptors expression, TGF-β, NF-κB and strogen receptor beta to elucidate the possible contribution of these mechanisms to the reno-protective effect of genistein. The lack of these data is one of our limitations in this study.
In our study, urine protein creatinine ratio was slightly lower in soy-genistein group than soy group, but this difference was insignificant. It seems we would observe significant difference in case of prolongation of the study. This observation supports the notion that the effects of soy are mainly due to its isoflavone, genistein.10,13 This study showed that adding genistein to soy protein enhances antioxidative status of kidney tissue, albeit insignificant. Some studies on the histopathology of nephrotic syndrome showed that soy diet in comparison to casein diet causes improvement in sclerotic and fibrotic sequels.13 Another research showed mesangeal and segmental proliferation with matrix expansion, capillary blockade, fibrosis with adhesion between glomerular coils and Bowman's capsule, also mononuclear cells infiltration in interstitial space in case of receiving casein diet. But glomerular damage and fibrosis were less in rats receiving soy protein.10 A research showed that soy replaced in animal protein improved glomerular filtration rate and glomerular hypertension. It also decreased the incidence of diabetic nephropathy.25 In the current study, adding genistein to soy protein caused profound improvement in histopathology of kidney. The improvement was so impressive that soy-genistein group resembled to normal group. Researches on the role of genistein in proliferation of malignant cell line showed that genistein induces apoptosis and suppression of cell proliferation through P53 pathway.26 Some researches supported the role of genistein in cell cycle inhibition specially breast and prostate cancer cell lines which are in consistent to our results on fibrosarcoma cell line WEHI-164.27 Another study on prostate cancer cell line showed inhibitory role of genistein on the activity of MMP-2 enzyme.28 Matrix metalloproteinases are inactive.29 Some factors like cancer cell lines, cytokines and inflammatory factors disturb their activity and balance.30
It is concluded from the results of current study that soy protein diet causes impressive changes in oxidative status markers and histopathological aspects of nephrotic syndrome. This study showed that adding genistein as an isoflavone to soy protein caused a greater improvement in this disease. We can conclude that the effects of soybean on nephrotic syndrome would be attributed to its isoflavone genistein.
ACKNOWLEDGMENTS
This study was supported by undersecretary of research in Tehran University of Medical Sciences (the grant number of 15438). We honestly appreciate Karen pharma & food supplement company (Tehran, Iran) for the donation of some food components.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 2. WEHI-164 fibrosarcoma cell line proliferation in different concentrations of genistein.
Figure 3. MMP-2 activity of WEHI-164 fibrosarcoma cell line in different concentrations of genistein.
Figure 4. Bands MMP-2 of WEHI-164 fibrosarcoma cell line in different concentrations of genistein.
Figure 5. Representative light microscopic view of histopathological slides of kidney in different groups.
Table 1. Ingredients of soy diet
Table 2. Urine protein creatinine ratio in group at the beginning and the end of study
Table 3. Score of microscopic findings of kidney histology in various groups
Figure 1. Concentration of malodialdehyde and of protein carbonyl, total antioxidant capacity and catalase activity in kidney tissue of four groups