Introducción: Los sistemas de hemodiálisis tienen capacidad trombogénica, por lo que se utiliza de forma rutinaria la anticoagulación. Su prescripción no se encuentra exenta de riesgos, a pesar de lo cual las recomendaciones respecto a la dosis pautada siguen basándose en criterios muy diversos. Métodos: Se realizó un estudio experimental aleatorizado y cruzado. Seis pacientes realizaron seis sesiones de hemodiafiltración posdilución con el dializador de polisulfona HF80® y anticoagulación habitual con nadroparina, y seis sesiones con el dializador AN69ST® de poliacrilonitrilo con una cubierta de heparina sin el uso de anticoagulación sistémica. Evaluamos cada hora el grado de coagulación del dializador y del circuito extracorpóreo mediante una escala visual y las variaciones en los parámetros de coagulación, entre los que se incluyó el factor anti-Xa. Nuestro objetivo primario fue valorar las variaciones en la actividad del factor anti-Xa en ausencia de diferencias en la tasa de coagulación masiva entre los dos grupos. Resultados: No se coaguló el dializador de forma completa o grado 4 en ninguna de las 36 sesiones realizadas con cada dializador. Se produjo una coagulación parcial del dializador inferior del 25% (grado 1-2) en 32 (88,9%) sesiones con AN69ST® y 35 (97,2%) con el dializador habitual, y superior del 25% (grado 3-4) en 4 (11,1%) sesiones con AN69ST® y en 1 (2,8%) sesión con el dializador con heparina. La coagulación del atrapaburbujas arterial no fue superior al 25% (grados 3 y 4) en ninguna de las sesiones estudiadas, y la cámara venosa en sólo 1 (2,8%) sesión con el dializador habitual y 3 (8,4%) con AN69ST® sin diferencias entre los dos dializadores. El valor del tiempo de tromboplastina parcial activada presentó diferencias a las dos horas entre ambas técnicas, relacionadas con la administración de la heparina de bajo peso molecular (33,3 ± 2,7 s con polisulfona y 27,5 ± 2,3 s en AN69ST®, p < 0,05), que continuaron siendo significativas al finalizar la sesión (29,8 ± 2,1 s con polisulfona y 27,2 ± 1,8 s con AN69ST®, p < 0,05). La actividad del factor anti-Xa fue máxima dos horas después de la administración de nadroparina, con diferencias entre ambos dializadores (0,46 ± 0,13 UI/ml en diálisis con polisulfona y 0,04 ± 0,04 UI/ml con AN69ST®, p < 0,005), para ir descendiendo en la determinación de las 4 horas (0,17 ± 0,12 UI/ml en diálisis con polisulfona y 0,02 ± 0,03 UI/ml en AN69ST®, p < 0,05). Un paciente fue excluido del estudio al presentar una reacción adversa caracterizada por prurito generalizado con el dializador AN69ST®, motivo por el que retiró en la primera sesión el consentimiento. Conclusión: Demostramos la baja trombogenicidad del dializador AN69ST® de forma que permite realizar sesiones de hemodiafiltración posdilución sin necesidad de anticoagulación sistémica, y sin aumentar la frecuencia de eventos de coagulación grave en comparación con el dializador HF80® junto a nadroparina y con menor riesgo de sangrado al no modificar la actividad del factor anti-Xa.
Background: Hemodialysis systems are potentially thrombogenic, so it is routinely used anticoagulation. Its prescription is with risks though which the recommendations regarding the scheduled dose are still based on very different criteria. Methods: We performed a randomized, crossover pilot study. Six patients underwent six sessions of post-dilution hemodiafiltration with polysulfone HF80® dialyzer and standard anticoagulation with nadroparin, and six sessions with heparin-coated poliacrylonitrile AN69ST® membrane without using systemic anticoagulation. Dialyser and the extracorporeal circuit clotting grade was evaluated through visual scale every hour and coagulation parameters like anti-Xa factor. Our endpoint was to assess anti-Xa activity without differences in the rate of massive clotting between the two groups. Results: No complete or grade 4 dialyzer clotting occurred in any of 36 sessions with each dialyzer. A partial lower 25% (grade 1-2) dialyzer clotting was in 32 (88.9%) AN69ST® sessions and 35 (97.2%) with the usual dialyzer and upper 25% (grade 3-4) in 4 (11.1%) AN69ST® sessions and 1 (2.8%) dialysis session with heparin. Arterial chamber blood coagulation was not greater than 25% (grade 3 and 4) in any of the studied sessions and the venous chamber in only 1 (2.8%) session with the usual dialyzer and 3 (8.4%) with no differences AN69ST® between the two dialyzers. The activated partial thromboplastin time at two hours showed differences between techniques related to administration of low molecular weight heparin (33.3±2.7 s with polysulfone and 27.5±2.3 s in AN69ST® p<.05) which remained significant at the end of the session (29.8±2.1 s with polysulfone and 27.2±1.8 s with AN69ST® p<0.05). Anti-Xa factor activity was maximal two hours after administration of nadroparin, with differences between the two dialyzers (0.46±0.13 IU / ml in dialysis with polysulfone and 0.04 ± 0.04 IU / ml with AN69ST®p<0.005) and went down after 4 hours (0.17±0.12IU / ml in dialysis with polysulfone and 0.02±0.03IU / ml in AN69ST® p<0.05). One patient in dialysis AN69ST® had an adverse reaction characterized by generalized pruritus and was excluded from the study, by withdrawing the consent in the first session. Conclusion: We demonstrate the low thrombogenicity of the AN69ST® dialyzer that allows post-dilution hemodiafiltration sessions without systemic anticoagulation, and without increasing the frequency of severe clotting events compared to HF80® dialyzer with nadroparin and with less risk of bleeding by not modifying the anti-Xa factor activity.
INTRODUCTION
Haemodialysis (HD) systems increase biocompatibility, but we have yet to be able to produce laminar flow which completely avoids system coagulation. The needles, lines, chambers, and membranes of the dialyser have a thrombogenic capacity, which necessitates anticoagulants in order to maintain the patency of the extracorporeal circuit.
During dialysis sessions in which anticoagulants are not administered, 5% of cases produce a notable coagulation of the dialyser,1 which translates into a loss of 150ml of blood occupying the lines and dialyser. For this reason, anticoagulation treatment is administered routinely in patients with no risk of haemorrhage, although substantial disparity exists regarding the criteria used for prescribing it.
Traditionally, unfractionated sodium heparin has been used for this purpose. The anti-coagulation properties of this compound are based on the conformational change of antithrombin III and the inactivation of coagulation factors, above all Xa. The effects of administering sodium heparin are immediate, with a half-life of 30 minutes to 2 hours. It is generally administered in boluses, a continuous drip, or through regional heparinisation, and is associated with secondary side effects such as haemorrhage due to excess doses, thrombocytopenia mediated by IgG-heparin immunocomplexes, and thrombocytopenia-associated thrombosis. Other undesirable effects that can appear include hypersensitivity, cutaneous necrosis, and osteoporosis.2
Low-molecular weight heparin (LMWH) provides an alternative for anticoagulation therapy. This drug is obtained by degrading the original heparin molecule into fractions of 4-6kDa. LMWH is used for its lower associated risk of bleeding, since it does not produce an anti-thrombin response. However, it does inhibit Xa, XIIa, and kallikrein, without producing any effect on thrombin, IX, or XI. The half-life of LMWH is longer than normal heparin, which allows for a single dose administered at the start of dialysis that is expressed in anti-Xa units. Although this drug is more expensive, it has fewer secondary side effects on platelet aggregation and thus produces fewer issues with bleeding. The European Best Practice guidelines recommend LMWH as the anticoagulant of choice for dialysis.3
Several different factors can increase the tendency of the circuit for coagulation, such as low blood flow, elevated haematocrit, and high rate of ultrafiltration; in addition, technical errors are an important cause of system coagulations. Isolated clots normally form during HD sessions using routine heparinisation. During the HD session, we can visually monitor signs of coagulation in the dialyser and circuit, such that, depending on the overall aspect of the fibres, we can adjust the anticoagulation dose for the following dialysis session. Laboratory analyses can also aid in assessing the coagulation state of the patient, but not of the circuit. We can measure the number of platelets and bleeding time, which serve to assess primary haemostasis and platelet function. Activated partial thromboplastin time (APTT) measures intrinsic coagulation activity and facilitates an effective control of unfractionated heparin levels. Finally, activated anti-Xa factor is a more sensitive test that allows for controlling coagulation during the use of LMWH.4
In the majority of HD units, these measurements are not normally made. The recommendations regarding dosage are based on body weight, comorbidities, bleeding, and type of HD.5,6 In patients with a high risk of haemorrhage, it is preferable to use HD without anticoagulation therapy, although the use of polyacrylonitrile dialysers with heparin coating is currently being introduced as an alternative method. The AN69ST® membrane design incorporates a polyethylenimine polymer that neutralises polyanionic groups, conferring the capacity for fixing heparin to the surface of the membrane through electric potentials. The AN69ST® membrane with heparinisation during the predialysis cleaning phase has low thrombogenicity and does not require saline solution flushes during its use on heparin-free HD. These haemocompatibility results show that the use of this type of membrane is safe and does not increase the risk of massive coagulation for the dialyser and extracorporeal circuit.7-10
We designed a study in patients on a haemodiafiltration (HDF) programme using AN69ST® dialysers without systemic anticoagulation therapy, with the goal of evaluating the variation in patient coagulation parameters, including anti-Xa factor levels; we evaluated the level of coagulation in the dialyser and extracorporeal circuit every hour using a visual scale. Our primary objective was to determine whether differences existed in anti-Xa levels in the absence of differences in terms of massive coagulation between the two groups.
MATERIAL AND METHOD
We carried out an experimental, randomised, crossed study including six patients with stage-5 chronic kidney disease on a periodic HD programme for at least six months before. We performed six consecutive sessions of post-dilution HDF using a high-permeability HF80® polyethersulfone filter (1.89m2) and anticoagulation using standard doses of intravenous nadroparin. In addition, six sessions were administered using AN69ST® dialysers using heparin-coated polyacrylonitrile membranes (2.2m2) without systemic anticoagulation therapy.
Patients and the order of treatment sessions were randomised. All patients signed an informed consent. The study was approved by the healthcare bioethics committee of the Castellon General Hospital.
Patients were selected based on the following inclusion criteria: age >18 years, with haemodynamic stability and a life expectancy longer than the predicted duration of the study. Each patient received dialysis treatment three times per week, and had serum albumin levels >3g/dl and haematocrit >30%. We also established the following exclusion criteria: active systemic infectious or inflammatory disease, uncured cancer, haemostasis disorders, or oral anticoagulant treatment at the time of the study. No patients were allowed to receive blood or blood derivative transfusions during the week prior to the study, during the study, or one week afterwards.
We included patients with native arteriovenous fistulas or prosthetic fistulas. We also attempted to minimise the differences in blood flow rates in each patient between types of HD. During the study period, neither the duration of HD sessions nor the type of solution used was modified, and the same conventional post-dilution or on-line HDF was used with programmed convection rates. We used the same dialyser during each period and the same anticoagulation dose during the normal dialyser period.
Prior to starting each dialysis session, the extracorporeal circuit was purged with 2l heparinised solution at 5000IU/l. Every hour, 100ml of saline solution was infused in order to observe the coagulation level of the dialyser and bubble trap.
On the sixth session, in each of the periods, we determined haemogram values in order to evaluate platelet count and haemostasis: prothrombin time, Quick index, APTT, and fibrinogen, and we also measured anti-Xa factor levels. The laboratory analyses were collected at the start of HD, after 2 hours, after ending the session, and prior to the following dialysis session or 44 hours later. We also measured plasma concentrations of urea, creatinine, glucose, sodium, potassium, calcium, phosphorous, total protein, albumin, uric acid, and C-reactive protein at the start of HD and at the end of each session.
The dosage for dialysis sessions was measured using the following formulas:
- Kt/V second generation Daugirdas: Kt/V= –Ln ([C2/C1] – [0.008*T]) + (4 – 3.5 * [C2/C1]) * UF/final weight.
- Kt/V corrected for rebound: Kt/Vr = Kt/V * (1 – [0.6/T]) + 0.03
Samples were sent for processing to the haematology laboratory at the Castellon General Hospital.
Samples were extracted for laboratory analyses after each session from 20-25ml of blood taken from the arterial branch after slowing the arterial pump to a flow of 50ml/min during one minute, according to the standard department protocols.
We evaluated circuit coagulation every hour using a photographic visual scale with 4 different coagulation levels in the arterial and venous bubble traps:
- 1 = normal.
- 2 = fibrous ring.
- 3 = clot formation.
- 4 = system coagulated.
The dialyser was inspected every hour until the end of each the dialysis session:
- 1 = normal.
- 2 = less than 25% of capillaries coagulated.
- 3 = 25-50% of capillaries coagulated.
- 4 = dialyser completely coagulated.
Statistical analysis
Prior to the study, we calculated the optimal sample size in order to achieve a statistical power of 80% and a 95% confidence interval for paired samples. The sample size was calculated by comparing the two means for anti-Xa factor obtained in studies from the medical literature.6,10 We also estimated the standard deviation of the mean at 0.1, based on previous studies. We defined Epsilon, which is the minimal difference between means that is considered of practical importance, at 0.2. We estimated that 4 patients would be needed, and taking into account the possible loss of 15% of the study sample, 2 more patients were added.
The risk of moderate or severe coagulation events with systemic heparinisation was defined as 5% of sessions, with a noninferiority margin of 10%.6,11,12 The unilateral α risk was 0.95. The number of sessions required (result) was 58. The possible loss of patients was estimated at 15%.
All statistical analyses were performed using SPSS software, version 11.5.
We used the Kolmogorov-Smirnov test to evaluate whether the study variables adhered to a normal distribution. For two-sample parametric variables, we used paired Student’s t-tests, and ANOVA tests for cases of more than two comparisons. For two-sample variables that did not adhere to a normal distribution, we used the Wilcoxon non-parametric test for paired samples, and repeated measures tests for cases of more than two comparisons. We also used Χ2 and Fisher’s exact tests to compare proportions between categorical variables. We evaluated bivariate relationships using a linear regression analysis, and calculated Pearson’s correlation coefficients in order to evaluate goodness of fit.
We considered a P-value <.05 to be statistically significant.
Ethical considerations
This study was carried out in accordance with the recommendations of the Helsinki Declaration of 1964, for the orientation of performing biomedical research in human subjects.
RESULTS
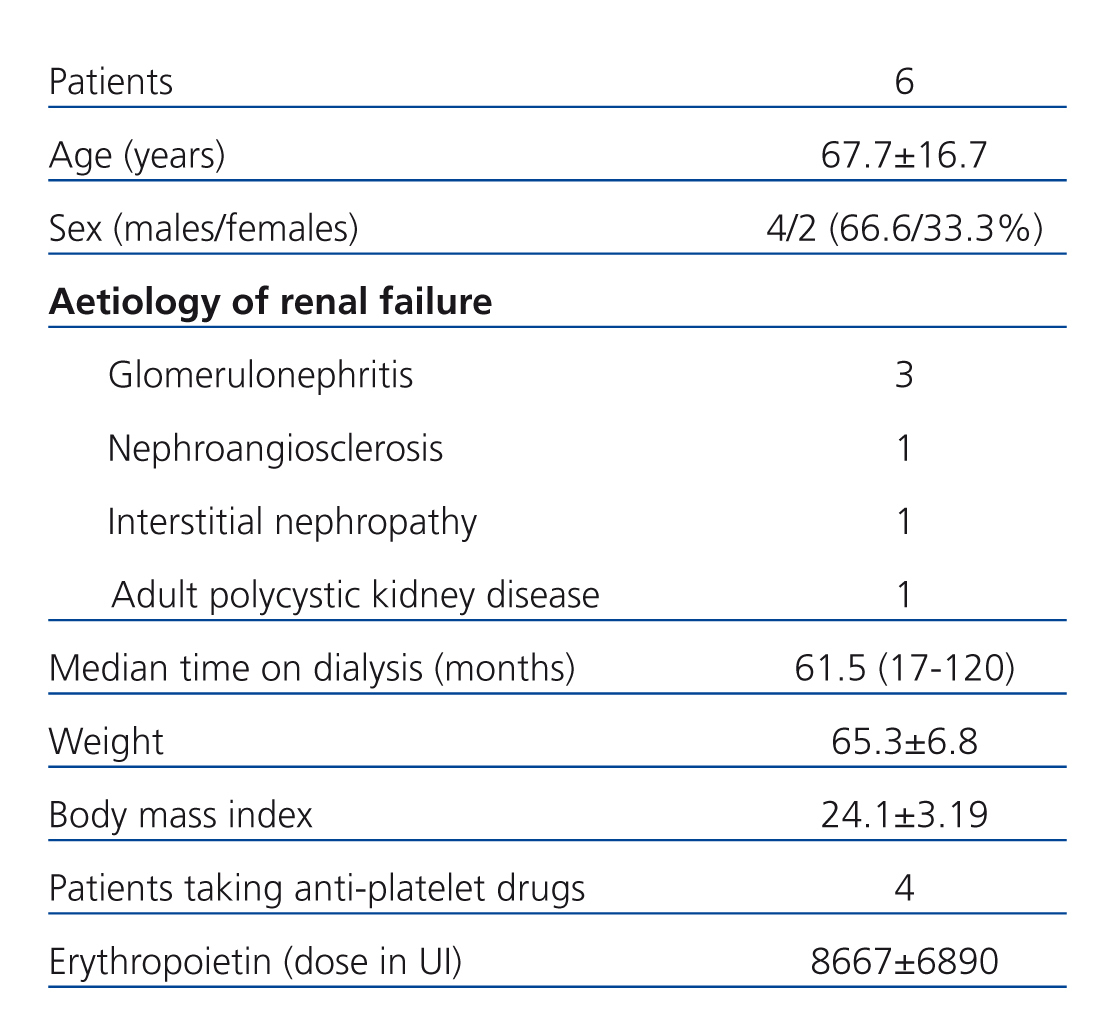
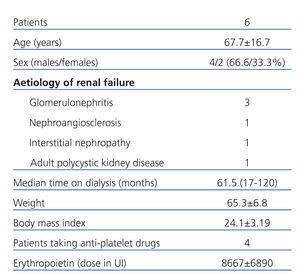
The demographic characteristics of the patients included in our study are described in Table 1.
We used systemic anticoagulation therapy only during sessions involving a conventional dialyser. The mean dose of nadroparin was 2500±547.7IU (38.5±8.2IU/kg).
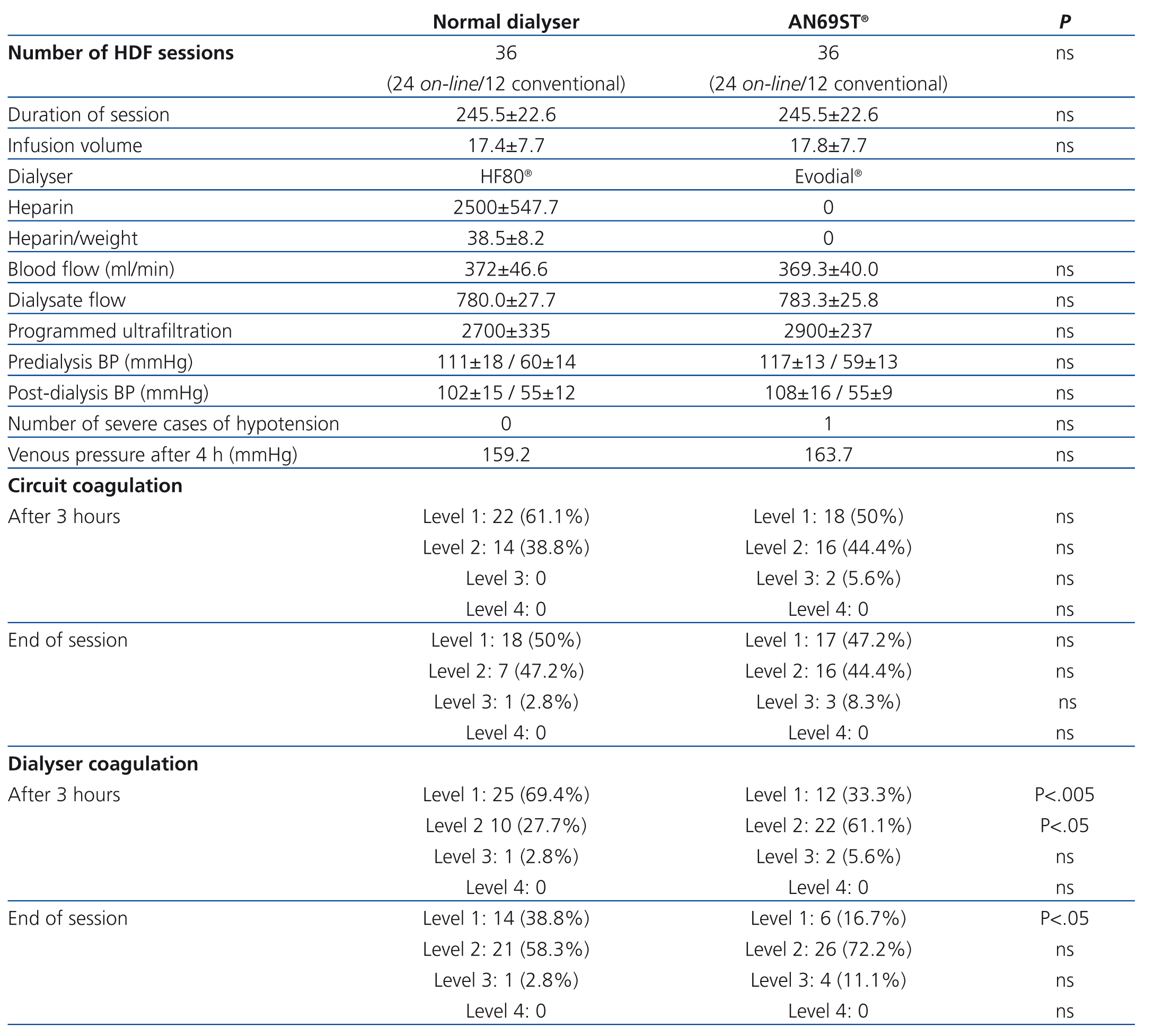
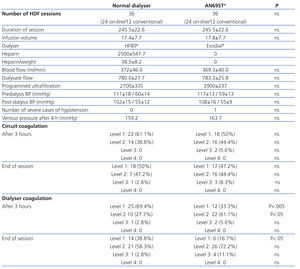
In our study, we did not observe differences in the distribution of coagulation levels between the two types of dialysers. No cases of complete or level 4 coagulation (>50% of the fibres in the dialyser coagulated) were observed in any of the 36 sessions using a conventional dialyser or the 36 sessions involving the AN69ST® system. Partial coagulation less than 25% (level 1-2) was observed in 32 (88.9%) of sessions using the AN69ST® system, and 35 (97,2%) sessions using the conventional dialyser, and greater than 25% (level 3) in 4 (11.1%) sessions without heparin and 1 (2.8%) session with the dialyser using heparin (Table 2).
Coagulation in the arterial bubble trap did not surpass 25% (levels 3 and 4) in any of the dialysis sessions, and this value was surpassed in the venous chamber in only one (2.8%) session using the conventional dialyser and in three (8.4%) sessions using the AN69ST®. There were no significant differences between the two dialysers.
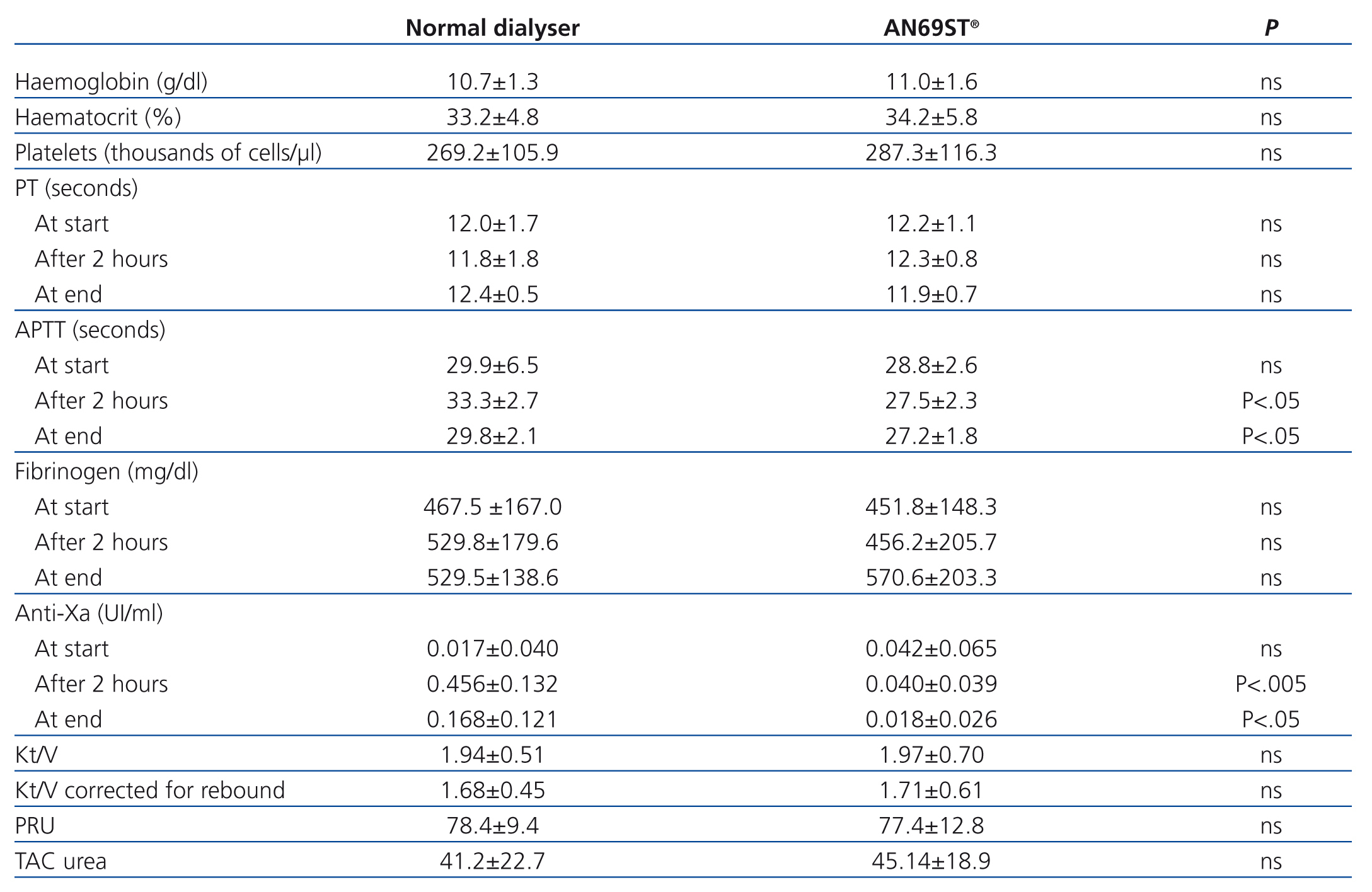
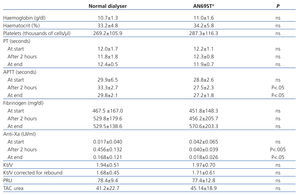
The APTT value after two hours was significantly different between the two techniques, and was correlated with the administration of LMWH (33.3±2.7s with polysulfone and 27.5±2.3s with AN69ST®; P<.05), and these differences continued to be significant at the end of the session (29.8±2.1s with polysulfone and 27.2±1.8s with AN69ST®; P<.05) (Table 3).
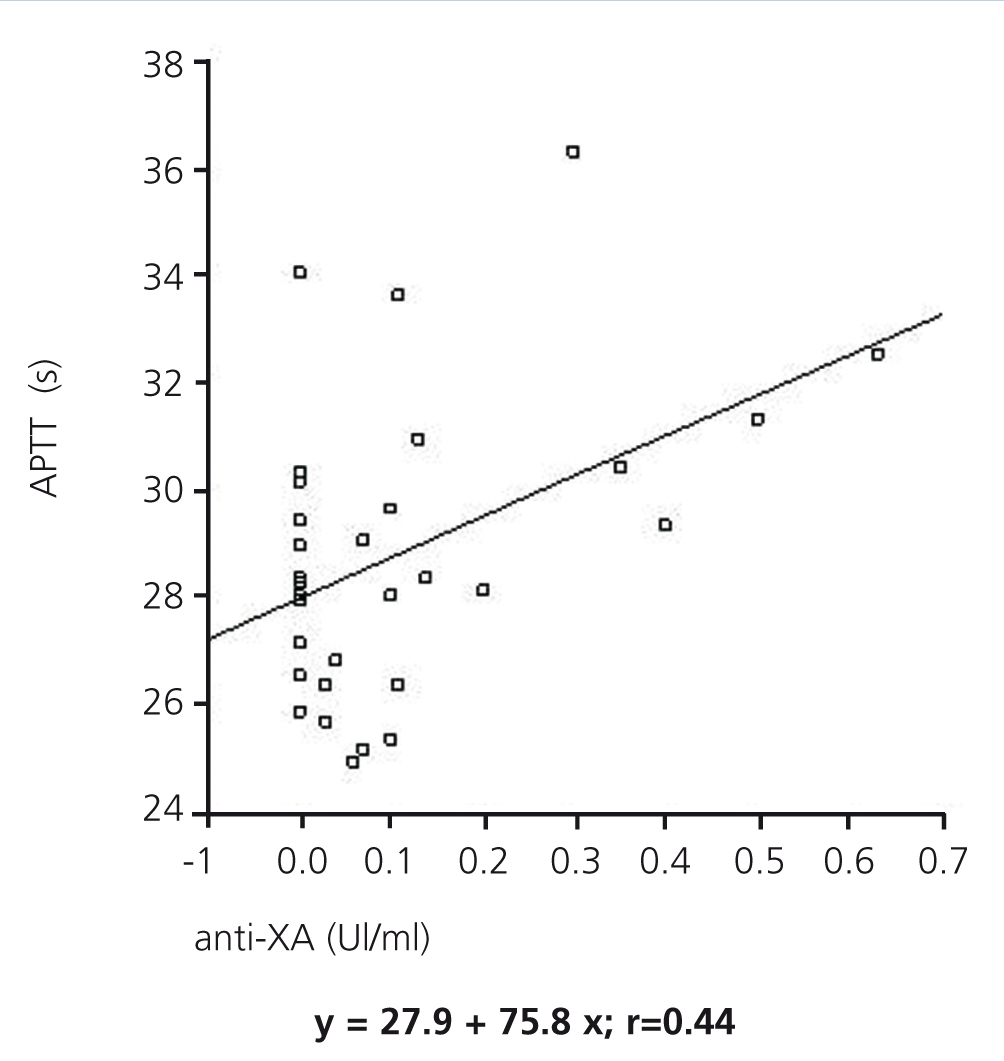
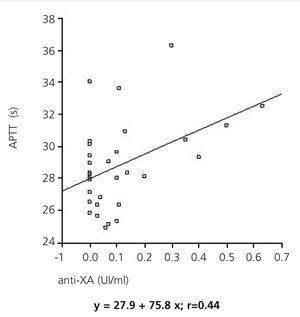
The anti-Xa factor value reached a maximum two hours after administering LMWH, with statistically significant differences between the two dialysers (0.46±0.13IU/ml in dialysis with nadroparin and 0.04±0.04IU/ml in AN69ST®; P<.005), and then decreased after 4 hours (0.17±0.12IU/ml in dialysis with nadroparin and 0.02±0.03IU/ml in AN69ST®; P<.05) for both modalities. A positive relationship was established between APTT and anti-Xa factor (regression equation: y=27.9+7.58x; r=0.44) (Figure).
The mean Kt/V value was similar for the two different treatments.
One patient on the AN69ST® dialyser developed abrupt hypotension with loss of consciousness. One patient on the AN69ST® dialyser was excluded from the study due to an adverse reaction in the form of generalised pruritus, for which the patient retracted the informed consent after the first session. Another patient developed nausea and general discomfort 15 minutes after starting a session using the AN69ST® dialyser, which was resolved with metoclopramide, and no further incidents occurred.
DISCUSSION
The type of dialyser and form of HD used are determining factors in the parameters of thrombogenicity present during dialysis sessions. The dialyser provides the largest surface area exposed to the blood. Flow control and circuit pressures are other factors that can be modified to minimise the activation of the coagulation pathway. In our study, we demonstrated a low thrombogenicity of the polyacrylonitrile AN69ST® dialyser, without needing anticoagulation therapy. The results were similar to those observed using a conventional high-permeability polysulfone dialyser with anticoagulation therapy in the form of LMWH using post-dilution HDF. This modality favours haemoconcentration throughout the dialyser capillaries, since the replacement infusion volume comes after ultrafiltration. These results are possible due to the presence of polyethylenimine as the polymer that covers and neutralises the negative charge of the polyacrylonitrile, allowing for the binding of unfractionated heparin molecules. The modifications made to the surface, along with the heparin coating, increase the biocompatibility of the dialyser membrane.13-15 This membrane pre-treatment minimises the binding of molecules involved in the contact activation of the intrinsic coagulation pathway.
Neither the dialyser nor the rest of the extracorporeal circuit were completely coagulated in any of the 36 sessions performed using the conventional dialyser or the AN69ST®. These findings are in accordance with previously published results, which situate the rate of complete system coagulation at 0.5%-2% of sessions.9,16 In sheep models, HD sessions were administered using the AN69ST® dialyser with heparin coating and without systemic anticoagulation therapy, with no system coagulation produced during 6-hour sessions. Under these same conditions, massive circuit coagulation was produced after 90 minutes using the AN69® dialyser without heparin coating.10 Studies in humans using the AN69ST® dialyser require an optimum level of sodium heparin (2000-3000IU), administered intermittently; with this prescription, partial coagulation was observed in 5/39 sessions using 2000IU heparin and 2/150 sessions with 3000IU heparin (P<.008). As regards the need for LMWH, 20mg of enoxaparin were administered, and no episodes of system coagulation were observed in the 30 sessions administered. When the dose was reduced to 10mg, partial coagulation was produced in 7/29 sessions.10
The experience gained using the AN69ST® dialyser without heparin and with adequate supervision resulted in only 1 case of massive system coagulation and 17 cases of partial or >25% coagulation in a sample of 66 sessions.10 Prior to the dialysis sessions, both groups were primed with a heparinised solution, such that the heparin would impregnate the membrane surface and maintain its anticoagulant effects during the session, without requiring systemic heparin administration. In a randomised crossed sample of 54 patients on HD without heparin, 6% of sessions were interrupted due to complete coagulation of the dialysers. A total of 9% of the sessions were with AN69ST®, and 13% with polysulfone developed partial circuit coagulation, with a persistent increase in venous pressure.11 Upon comparing the AN69ST® dialyser with another polysulfone dialyser using a visual scale, in patients in which the anticoagulation therapy had been decreased by 50%, no differences were observed in coagulation in the venous bubble trap.15
Anticoagulation therapy in HD implies a risk for active bleeding in dialysis patients, both as a new condition and in terms of worsening pre-existing issues. Standard systemic heparinisation has been associated with a risk of haemorrhagic complications that can occur in as many as 26% of all sessions.17 Several different alternatives have been used in patients with a high risk of bleeding. Intermittent flushes with saline solution do not appear to improve the visual signs of circuit coagulation or intravascular coagulating activity parameters in stable patients.18 It is used in 90% of patients in intensive care units, with only 2% of cases developing complete circuit coagulation.16 Regional heparinisation or small controlled doses of heparin have been used in different studies involving patients with a high risk of bleeding.19 Using the AN69ST® dialyser allows for reducing the dose of unfractionated heparin administered in HD by 50%, without increasing the risk of circuit coagulation.7
In our study, we observed a positive relationship between APTT and anti-Xa factor levels. Both values increased two hours after starting heparin administration. Anti-Xa values remained stable during the entire session with the AN69ST® dialyser, whereas in sessions with systemic heparinisation, these levels increased, reaching values that avoid circuit coagulation but that alter the coagulation state of the patient. In the last two hours, levels decreased, but remained above normal at the end of the HD session. Specific haemostasis values have been defined that reduce the risk of circuit coagulation. APTT must be maintained >40s and anti-Xa factor >0.2mIU/ml, based on the results from visual inspection of the dialyser and chambers.10 In sessions with nadroparin, despite using doses of 38.5±8.2IU/kg, anti-Xa levels remained close to 0.2 at the end of the HD session, which implies a risk of haemorrhage that is not reflected in the value of APTT, which decreased to levels similar to those at the start of the session.
Other studies using the AN69ST® have encountered various factors that serve as measures of thrombogenicity during the HD session, such as prothrombin fragment 1+2 as a marker for the vascular formation of thrombin, thrombin-antithrombin III complexes, or B-thromboglobulin as a marker for platelet activation.18
CONCLUSIONS
In our study, we demonstrated a low thrombogenicity of the AN69ST® dialyser, such that we were able to perform sessions of post-dilution HDF without requiring systemic anticoagulation, without increasing the frequency of severe coagulation events, and with a lower risk of bleeding by not modifying the anti-Xa factor.
Conflicts of interest
The authors have no conflicts of interest to declare.
Table 1. Demographic characteristics of the study population
Table 2. Technical characteristics of dialysis sessions
Table 3. Patient results (coagulation and dialysis efficacy parameters)
Figure 1. Association between APTT and anti-Xa factor during the 4 hours of treatment