Encapsulating peritoneal sclerosis (EPS) is an uncommon but severe complication among patients on peritoneal dialysis (PD). This report describes three patients from our centre who were diagnosed of EPS collected during a 20-year period.
Case 1A female patient with Laurence-Moon-Biedl syndrome. Haemodialysis (HD) was started at the age of 11. She received her first renal transplant in the following year, which failed due to thrombosis of the vein graft. Two years later, she was started on PD because of difficulties with vascular access. The patient developed several peritonitis and severe hyperparathyroidism. Her peritoneal transport was medium–high at baseline. After 12 years of dialysis, this patient had a new onset of peritonitis, associated with tunnel infection and peritoneal ventral hernia in the catheter site. Ultrasounds revealed sclerosis and peritoneal calcification with compressed abdominal viscera. The patient received antibiotics, the catheter was withdrawn, and she was started on HD. She later developed acute ischaemia in the right lower limb as a result of femoral–popliteal obstruction. Surgery was not considered due to the poor patient's clinical condition, and she died soon after.
Case 2A male patient diagnosed of rapidly progressive glomerulonephritis with basal glomerular anti-membrane antibodies was treated with steroids, cyclophosphamide, and plasmapheresis with no positive results. In his 5 years of PD, the patient developed 10 episodes of peritonitis, 8 of which were due to Staphylococcus aureus. The last peritonitis was caused by Candida albicans; and for this reason, the catheter was withdrawn and the patient started HD.
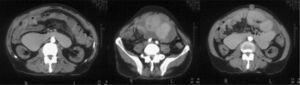
Peritoneal transport was originally medium–high, and finally became high. Six months later the patient developed abdominal pain and a mass effect was reflected in the suprapubic region. Peritoneal thickening, pelvic fluid collection with septa comprising loops, and large gastric and intestinal dilatation were observed in a CT scan (Fig. 1). Surgery was not considered and the patient was hospitalised several times because of intestinal occlusion; his general condition became progressively worse and he died soon after.
Case 3A male patient who had focal segmental hyalinosis which was refractory to steroids, cyclophosphamide, and vincristine. He started PD in 1990. During the following 11 years he received 3 renal transplants, with a total dialysis-free time of 5 years. He underwent parathyroidectomy due to severe hyperparathyroidism.
Peritoneal transport remained medium–high with a tendency to increase. Ultrafiltration decreased over time and the need for osmotic solutions was greater. CA-125 was measured in the peritoneal effluent several times and a gradual decrease was observed over time. The patient had 12 peritonitis, all of them caused by Gram positive germs. HD was indicated, but the patient still tried to avoid it by all means; he finally started HD in 2011.
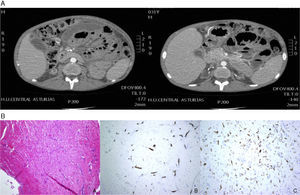
Six months later, he was admitted with pain and abdominal distension. A CT scan of the abdomen (Fig. 2A) showed gastric and duodenal dilatation with free loculated fluid and oedematous bowel. A peritoneal biopsy revealed proliferation of small vessels, inflammatory cellularity, and fibrosis (Fig. 2B). After EPS was diagnosed, treatment with prednisone and tamoxifen was started. The size of the collection had decreased after six months, and no loop dilatation was observed. The patient did not developed more complications. He is currently receiving HD and tamoxifen.
DiscussionThe incidence of EPS in our Nephrology service was 0.47% lower compared to other case studies where it ranges between 0.7 and 7.3%.1–3 It is an uncommon but severe complication characterised by a high mortality rate (two of our patients died). Pathogenesis is unclear, and constant peritoneal inflammation associated with glucose and glucose-degradation products is thought to enhance peritoneal permeability to certain substances, including fibrin, thereby promoting fibrosis; in addition, a second stimulus, such as peritonitis, abdominal surgery, bioincompatibility of solutions, or long time in PD,4–7 may accelerate progression or ultimately trigger EPS.2 Some of these have been clearly implicated in the development of EPS in our patients.
Finally, both the interruption of the washout and the build-up of profibrotic factors,8 as well as the use of anti-calcineurin drugs, give rise to increased fibrosis.9
Clinical manifestations of EPS consist of signs and symptoms of bowel obstruction.10 X-rays show an image of “cocoon” (encased loops due to fibrosis), adhesions, air-fluid levels, and peritoneal calcification. Diagnosis is confirmed by histological findings: mesothelial detachment and thickened peritoneum with fibrin deposition, inflammatory infiltrates, capillary angiogenesis, and calcification.10
No biochemical markers or X-ray examinations are able to screen patients at risk of EPS. Low CA-125 and high IL-6 in the peritoneal effluent have been suggested as prognostic factors.11 Our third patient developed a progressive decrease during PD.
Prevention is the treatment cornerstone, including stronger and earlier options, such as the use of biocompatible solutions and early screening. Immunosuppresants, particularly corticosteroids and tamoxiphen, are the treatment of choice for the management of inflammation as a result of their anti-fibrotic effects. In advanced stages, adhesions and encased loops can be released by surgery, and yet, even though intestinal obstruction is reverted, peritoneal impairment is not halted.4
Please cite this article as: Merino Bueno C, del Rio García L, Bande Fernández JJ, García R, Hidalgo Ordoñez C, Rodríguez-Suárez C, et al. Esclerosis peritoneal encapsulante: revisión de 3 casos. Nefrologia. 2015;35:588–590.