Emphysematous pyelonephritis (EPN) is a rare necrotizing condition of the kidney, and is particularly rare in renal transplant recipients. It is characterized by the presence of gas in the renal parenchyma, the perirenal space, or the urinary tract as a result of an infection. Infection is generally caused by gas-producing microorganisms, including Escherichia coli (over 80% of cases) or Klebsiella pneumoniae, due to mixed fermentation of glucose.1 Over 80% of cases have been described in diabetic patients. Urinary tract obstruction is a major risk factor of EPN, and women are affected more often in a 4:1 ratio.2 Mortality from EPN is primarily attributable to septic complications, with almost 78% of cases reported by the late 1970s. However, the improvement in management techniques has reduced this figure to 21% over the last two decades.3
The clinical course of EPN is typically severe and rapidly progresses to sepsis with multiple organ failures. CAT scans are considered the gold standard for diagnosis and staging.1
The time between graft placement and the development of EPN among renal transplant recipients as reported in the literature ranges from two weeks to 15 years.4,5 Al-Geizawi et al.2 suggest a classification of EPN into renal grafts based on CAT findings. This will require prospective validation however, but it is an alternative to previous classifications of EPN in native kidneys. This classification would help guide medical or surgical treatment, depending on the indication.
Clinical caseThe patient was a non-diabetic and non-hypertensive 50-year-old patient with a history of renal failure secondary to reflux-associated chronic pyelonephritis, and a history of four renal transplants (1992, 2001, 2006, and 2010). Vascular endoprosthesis and intraperitoneal placement were required in the last renal graft placement in the left iliac fossa (LIF). Graft loss resulted from: (1) suspected chronic nephropathy of the graft, with three episodes of acute rejection with transplantectomy in 1999; (2) early obstructive uropathy with transplantectomy one year later; (3) thrombotic microangiopathy associated with humoral rejection, and (4) suspected humoral rejection. As a consequence, haemodialysis was restarted in November 2011, immunosuppressants had been tapered and oral corticoids had been continued.
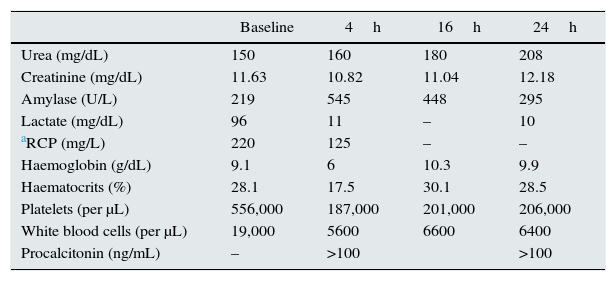
The patient presented at the emergency department with haemorrhagic shock resulting from rectal bleeding. There were no previous fever or symptoms. The case also had previous haemodialysis sessions with no incidents. Investigations revealed: generalized pallor, diaphoresis, afebrile, and poor performance status with haemodynamic instability (BP: 82/56mmHg, HR: 133bpm, and SaO2 70%). Mild abdominal pain in the mesogaster, with no signs of peritoneal irritation was observed. Rectal examination had shown fresh blood. There was an unpainful renal graft in LIF. Emergency tests revealed severe anaemia with positive sepsis markers (Table 1).
Tests evolution.
| Baseline | 4h | 16h | 24h | |
|---|---|---|---|---|
| Urea (mg/dL) | 150 | 160 | 180 | 208 |
| Creatinine (mg/dL) | 11.63 | 10.82 | 11.04 | 12.18 |
| Amylase (U/L) | 219 | 545 | 448 | 295 |
| Lactate (mg/dL) | 96 | 11 | – | 10 |
| aRCP (mg/L) | 220 | 125 | – | – |
| Haemoglobin (g/dL) | 9.1 | 6 | 10.3 | 9.9 |
| Haematocrits (%) | 28.1 | 17.5 | 30.1 | 28.5 |
| Platelets (per μL) | 556,000 | 187,000 | 201,000 | 206,000 |
| White blood cells (per μL) | 19,000 | 5600 | 6600 | 6400 |
| Procalcitonin (ng/mL) | – | >100 | >100 |
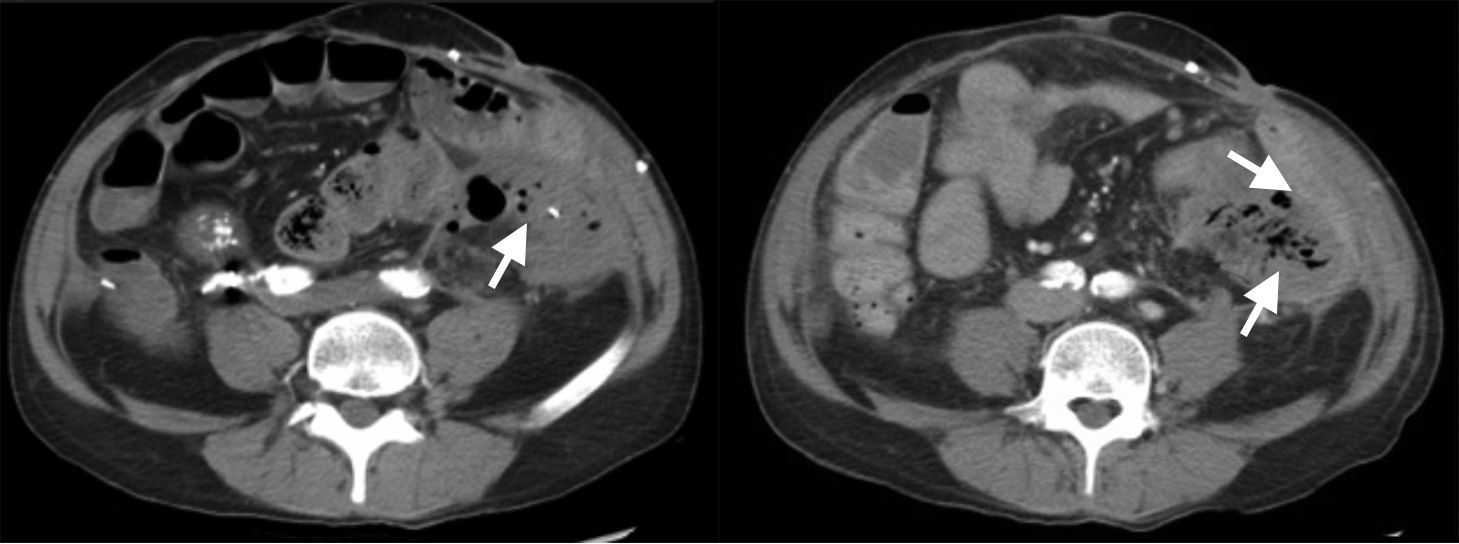
The patient was admitted into the intensive care unit (ICU) with volemic replacement of 7 concentrates of red blood cells. No bleeding focus was observed in the colonoscopy, the abdominal CT angiography, and the aortography. Abundant gas was seen in the interior of the LIF renal graft (Fig. 1), leading to the initiation of broad-spectrum antibiotics (piperacillin/tazobactam). Following ICU discharge, the patient was referred to Nephrology, and a new colonoscopy was still unable to reveal the origin of bleeding. Persistent gas was confirmed by ultrasound at the level of the renal graft.
A LIF renal graft transplantectomy showed an unstructured kidney with abscess formation. The pathology report revealed ischaemic necrosis with renal artery thrombus. K. pneumoniae and Klebsiella oxytoca were isolated from cultures.
The patient made good progress following the procedure. Some days later a new episode of melena occured and an arteriovenous fistula was evidenced from the distal mesenteric artery, as seen in the CT angiography. Medical nephrectomy was performed with a satisfactory outcome.
The patient died after two new episodes of rectal bleeding and anaemia.
A case of EPN is described in a patient with a non-functioning renal graft following the analysis of an episode of digestive haemorrhage. Typical imaging consistent with this disease is described in the CAT scan of the case. Treatment included transplantectomy and wide-spectrum antibiotics. There was histological evidence of injuries consistent with EPN in the patient. Two microorganisms commonly reported to be isolated in the literature, K. pneumoniae and K. oxytoca, were found in cultures of the pathological specimen of this case.6
Companion diagnostics must be made with the presence of renal abscesses, xanthogranulomatous pyelonephritis, and renal tuberculosis.7
Even though our patient was not diabetic, the history of immunosuppression due to multiple previous renal transplants may become a risk factor associated with EPN.
This kind of clinical cases should be reported to further investigate their epidemiology and clarify the pathogenic aspects guiding the selection of optimal treatment for future cases.6–9
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hernández-Vargas H, Sierra-Carpio M, Gil-Catalinas F, Bello-Ovalle A, Beired-Val I, Pimentel-Guzmán GI, et al. Pielonefritis enfisematosa en trasplantado renal. Reporte de un caso. Nefrologia. 2016;36:184–186.