To the Editor:
There are very few reports of haemophagocytic syndrome and infection by histoplasmosis in transplanted patients.1 We present the cases of two renal transplant patients who, after suffering acute organ rejection and therefore needing more immunosuppression, developed disseminated infection of histoplasmosis. Their clinical and laboratory data were compatible with haemophagocytic syndrome.
Case 1. 30-year-old female with chronic end-stage renal failure (CRF) secondary to hyperoxaluria. The patient receives a renal transplant from a deceased donor; alemtuzumab induction and maintenance with mycophenolate-cyclosporine. Twelve moths later the patient presents acute rejection 1B and cyclosporine is replaced by tacrolimus. Six months later, the patient comes into a consultation due to fevers, arthritis and a generalized maculopapular rash. Clinical analysis report leucocytes: 1400/mm3, platelets: 100,000/mm3, creatinine: 4,3mg/dl, blood urea nitrogen (BUN): 75mg/dl, total bilirubin: 2,41mg/dl and direct: 2,15mg/dl, gamaglutamil-transferase: 552U/l, oxaloacetic transaminase: 839U/l, glutamic pyruvic transaminase: 348U/l, ferritin: 372,522ng/ml, triglycerides: 462mg/dl, fibrinogen: 213, chest tomography with ground glass pattern and areas of consolidation, abdominal ultrasound with splenomegaly. Polyculture is performed; we start to administer meropenem, linezolid and doxycycline. Studies for cytomegalovirus (CMV), toxoplasmosis, human immunodeficiency virus (HIV), hepatitis A, B and C, rickettsia, leptospirosis, tuberculosis, cryptococcus, Epstein-Barr virus (EBV) all returned negative. Patient requires ventilator support and renal replacement therapy (RRT) due to further clinical downturn. Three days later, haemocultures and myelogram isolate intracelullar yeasts compatible with histoplasm. We start to administer amphotericin B, and then intraconazole. The patient improves, with partial recovery of renal function; final creatinine level: 2.3mg/dl. RRT is suspended and immunosuppression resumes.
Case 2. 41-year-old female with end-stage CRF of unknown aetiology and renal transplant from donor deceased 4 years prior. She received induction with alemtuzumab and tacrolimus-mycophenolate maintenance. Four months before, she presented cellular acute rejection 1A and required an increase of immunosupressive medication. She is hospitalized due to fifteen days of fever, ulcers in pubic areas and maculopapular rash. The paraclinical exams show haemoglobin: 7.58g/dl, haematocrit: 22.5%, leukocytes: 2520/mm3, platelets: 7000/mm3, creatinine: 5,8mg/dl, BUN: 68mg/dl, ferritin: 28,805ng/ml, lactate dehydrogenase: 314U/l, triglycerides: 352mg/dl, fibrinogen: 305, normal thorax radiography and abdominal ultrasound with hepatosplenomegaly. Studies are run on the patient to test for dengue, toxoplasma, haemoparasites, HIV, hepatitis A, B, and C, EBV, rickettsia, leptospira, cryptococcus, all negative. Polyculture is performed and antibiotic treatment begins with unfavourable evolution which progresses quickly into organic multisystemic failure. Patient then requires RRT and ventilator support. Three days later the haemocultures report histoplasma, that same day the patient passed away.
DISCUSSION
Histoplasma capsulatum is a dismorphic fungus that causes endemic systemic mycosis in certain areas of America, Africa and Asia.2 It is a granolumatous disease that affects mainly the lungs and the immune system. It is found in caverns and abandoned construction sites, especially where deposits of birds and bat faeces can be seen.3
The infection is acquired by inhaling their spores.2.The severity of the disease depends on the number of spores inhaled and the immune state of the host organism; in healthy patients the infection is asymptomatic or similar to a flu syndrome.4-6 However, in patients with immune system deficits the infection can lead to haematogenous spread involving the lungs, liver, spleen, bone marrow, and central nervous system (CNS).
In patients with renal transplant, the clinical frame is not specific.6 50% of patients develop respiratory symptoms and 75% disseminated histoplasmosis5, characterized by the involvement of at least two organs.4 25% to 60% of patients have hepatosplenomegaly. Frequently there is septic shock.5 The CNS manifestation is observed on less that 10% of cases6; other symptoms can include paniculitis, orofacial complications, ileal perforation and meningitis.1
Although histoplasmosis in transplanted patients is uncommon, and it has been prevalent in patients with hepatic or renal transplant,6 the reported incidence is between 1-5 cases in 3436 transplant patients and for a maximum period of 75 years.1,4
When it comes to the diagnosis, the culture, though sensitive and specific, requires several weeks for correct identification. Though the antibodies are not reliable, the detection of the urine antigen for this fungus is one of the fastest and most sensitive methods: it finds 90% of patients with disseminated infection and 75% with acute pulmonary histoplasmosis.5
With the appropriate therapy, the prognosis for the disseminated form of the infection is excellent given that mortality in the absence of treatment is 80%.5 The initial treatment for severe infections, besides decreasing inmunosuppresion,6 is amphotericin B dose of 1mg/kg per day until observed improvement of the disease, usually for 1-2 weeks, continuing with 200mg itraconazole 2 times a day for 12 months. In moderate infection, we start with itraconazole 3 times a day during 3 days as loading doses, followed by 200mg, twice a day for 12 months.4,6
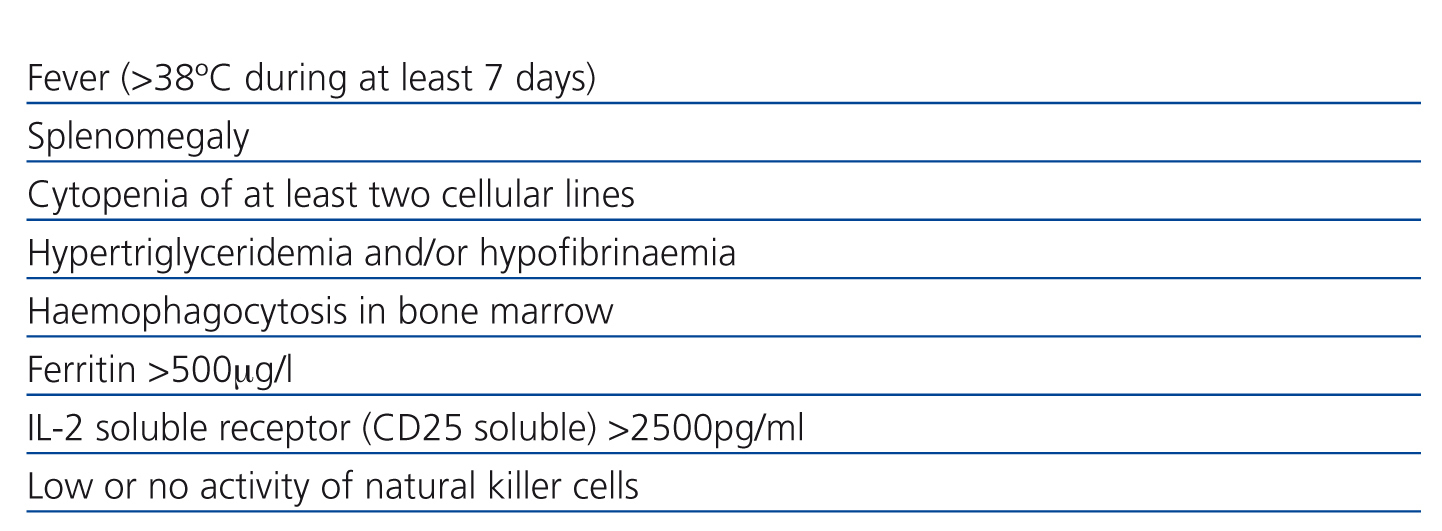
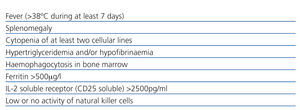
Haemophagocytic lymphohistiocytosis (HL) or haemophagocytic syndrome is characterised by an extensive inflammatory response to a variety of infections or immune system abnormalities,7-9 with excessive cytokine production which leads to activation of T lymphocytes and macrophages in liver, spleen and bone marrow. For its diagnosis, at least five criteria are required (Table 1). Other findings include lymphadenopathy, rash and neurological symptoms. In suspected cases, bone marrow biopsy can confirm diagnosis with the presence of haemophagocytosis in red blood cells and other blood elements;9 however, a negative result does not exclude the diagnosis. A valuable sign of this disease, though not specific, is ferritin: the higher its level, the greater the possibility of having the syndrome. On this report the diagnosis of haemophagocityc syndrome was performed with five clinical criteria because we could not find phagocytes in the bone marrow.
HL may be inherited but the most commonly acquired form is caused by immunodeficiencies, haematological malignancy or secondary to infectious processes especially EVB, CMV and fungus.9 Mortality in transplanted patients nears the 50%. In case it is associated to histoplasmosis, optimal treatment is based in support measures and the adequate anti fungal therapy.1,7,9
This revision seeks to draw attention to the possible association between disseminated histoplasmosis and haemophagocytic syndrome in renal transplant patients which may induce a multi organic failure, a fact that increases its lethality.11 Timely diagnosis and treatment could improve the final prognosis.9
Conflicts of interest
The authors have no conflict of interests to declare.
Table 1. Diagnostic criteria for haemophagocytic syndrome