La enfermedad celíaca se produce por la interacción entre el gluten contenido en los cereales y varios factores genéticos y autoinmunes. Aunque las manifestaciones clínicas predominantes son digestivas, se han descrito varias manifestaciones sistémicas. También se ha asociado con varias enfermedades renales, entre las que predominan las glomerulonefritis. Describimos el caso de una paciente con una enfermedad celíaca aparecida tras su primera gestación que de forma simultánea presenta proteinuria nefrótica y microhematuria, con sustrato morfológico de nefropatía membranosa. Tras recibir tratamiento con medidas conservadoras (IECA, estatina) y dieta sin gluten, se aprecia mejoría de la clínica digestiva, y desaparición de los anticuerpos antitransglutaminasa tisular tipo IgA y de la proteinuria. Revisamos la relación entre enfermedad celíaca y nefropatía membranosa y el papel de la dieta libre de gluten en el control de las mismas.
INTRODUCTION
Coeliac disease (CD) is a relatively common entity with a prevalence in this country of 1:250 to 1:500, depending on which diagnostic criteria are employed.1 Genetic factors (HLA-DQ2 and/or DQ8 antigens) are involved in its pathogenesis, as well as environmental factors, and there are changes in humoral and cellular immunity responses to antigens contained in cereals (wheat, barley, rye and oats) which contain a protein called gluten. The peptides derived from gliadin (the alcohol-soluble fraction of gluten) possess many toxic components, which contain various antigens implicated in its pathogenesis.2 CD can appear at any age, with symptoms of malabsorption, which are more or less evident and may be associated with other autoimmune diseases. An increase in renal diseases,3 the most prominent of which are forms of glomerulonephritis, especially nephropathies with mesangial IgA deposits, has also been reported.4
We describe a case of classical CD, which appeared after the patient¿s first pregnancy and was associated with nephrotic proteinuria secondary to membranous nephropathy (MN). After receiving treatment based on conservative measures (ACEI, statins) and a gluten-free diet, there was an improvement in the patient¿s digestive symptoms, a disappearance of circulating IgA anti-tissue transglutaminase antibodies and a parallel decrease in proteinuria. We investigated the relationship between CD and MN, as well as the role of a gluten-free diet in its control
CASE REPORT
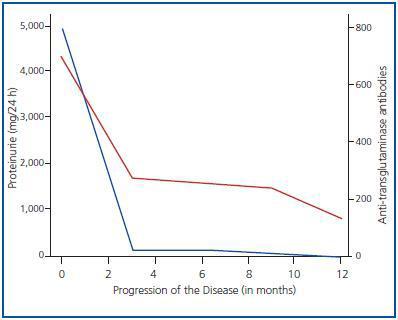
A female patient, aged 35 years, with no relevant medical history, presented symptoms, including abdominal pain, diarrhoea and constitutional syndrome, some weeks after giving birth to her first child; there were no complications during the pregnancy. The patient was examined in the Gastroenterology Department, where she was diagnosed with CD on the basis of the following diagnostic criteria: 1) high anti-transglutaminase antibody levels and 2) an intestinal biopsy which revealed villar atrophy in the duodenum and crypt hyperplasia. Shortly after the onset of these symptoms, she developed polyarthralgias affecting middle joints, predominantly of the wrists and feet, together with paresthesias and cutaneous lesions with pruriginous micropapules. She was referred to the Nephrology Department upon detection of proteinuria (+++). Results of Physical Examination: blood pressure 104/76mm Hg, BMI 27, minimal oedema of the lower limbs and papules on elbows and arms. Analytical findings: normal haemogram and coagulation, creatinine 0.9mg/dl, total cholesterol 238mg/dl, triglycerides 104mg/dl, total protein 6.5g/dl and albumin 3.6g/dl. Immunological assays: ANA, anti-DNA, ANCAs, C3, C4, anti-cardiolipin antibodies, lupic anticoagulant and cryoglobulins negative or normal. Serological tests: HBV, HCV and HIV negative. IgA antitissue transglutaminase antibodies positive: 800U/ml (normal < 7U/ml). Urine: proteins 4.4g/day and sediment with 5 RBC/HPF. The renal echography was normal. Given the persistence of the nephrotic proteinuria over a period of several weeks, a percutaneous renal biopsy was performed, the result of which revealed stage 2 MN with no vascular or interstitial lesions. Treatment consisted of a gluten-free diet and other conservative measures, including statins, aspirin at anti-aggregant doses, enalapril and losartan, to which there was no initial response. However, the follow-up results at 12 months were negative for anti-transglutaminase antibodies and there was complete remission of the proteinuria, as indicated in figure 1. During this time there were no other indications of systemic disease, except an increase in TSH levels to 6.6μU/ml, with a T41 level of 1.1ng/dl, and negative results for anti-thyroglobulin and anti-microsomal antibodies.
DISCUSSION
In this case we describe a patient who simultaneously presented CD and MN, an association which has been described very little in the literature. It is very likely that both entities have a common pathogenesis and require similar treatment.
CD is an autoimmune disease, which appears after exposure to environmental antigens (the gliadin contained in gluten) in patients with a genetic predisposition. For reasons which are unknown, certain components of gliadin provoke a change in the immune response in the intestinal wall, which is associated with local inflammation and the release of tissue transglutaminase, an enzyme produced by inflammatory and endothelial cells, and fibroblasts. Tissue transglutaminase increases the antigenicity of gluten peptides, stimulating T cells, which leads to villar atrophy and, consequently, malabsorption syndrome.1,2 CD can appear at any age and, sometimes, there are few symptoms, although it starts off with diarrhoea, abdominal pain and distension, vomiting, flatulence and anorexia. Its non-digestive manifestations include: iron deficiency anaemia, polyarthritis, growth retardation, osteoporosis and neurological symptoms. A biopsy of the duodenum-jejunum junction is indispensable for the diagnosis of CD. Atrophy, shortening of the villi and crypt hypertrophy in the small intestine are not specific to, but are very characteristic of this disease. In addition, the presence of high levels of IgA anti-tissue transglutaminase antibodies is useful for diagnostic purposes and for evaluating the progression of the disease, as their disappearance parallels clinical improvement; it is estimated that their sensitivity and specificity are 93.1 and 93.6%, respectively.1,5 Therefore, our patient met the three criteria required to confirm a diagnosis of classical CD: villar atrophy, symptoms of malabsorption and the disappearance of symptoms after following a gluten-free diet.
CD is associated with other autoimmune diseases, such as dermatitis herpetiformis, IgA deficit, Sjögren¿s syndrome, Addison¿s disease, type I diabetes, thyroid diseases, systemic lupus and hepatitis.1,6 The patient described in this case study had skin lesions, possibly atopic dermatitis, which is also associated with CD, that were not shown conclusively to be related to the disease. Moreover, its onset was associated with polyarthralgias, with no evidence of systemic lupus, possibly owing to symptoms of osteoarthritis, which has also been reported in CD. Moreover, patients with CD exhibit a greater prevalence of renal diseases. In an epidemiological study conducted in Sweden, Ludvigson et al.3 described an increase in the number of cases of chronic glomerulonephritis and advanced renal insufficiency in patients with CD and this risk is greater when the diagnosis is made in adults.
Although the incidence of renal disease in the CD patient population is low and, vice versa, the presence of CD in patients diagnosed with glomerulonephritis is minimal, this ssociation has pathogenic aspects that are common to both conditions and which can affect treatment. Isolated cases of CD linked to nephrotic syndrome have been documented. Giménez et al.7 reported a series of five children with nephrotic syndrome, who at some point developed CD; in the renal biopsy, performed on two of them, lesions with minimal changes or mesangial glomerulonephritis with IgM deposits were detected. In our opinion, it is best to rule out CD, which is more or less masked, in patients with glomerular nephropathies, given how easy it is to establish anti-tissue transglutaminase antibody levels and the therapeutic implications this entails. By far the main association between CD and glomerular disease is IgA nephropathy, which occurs in 3% of CD patients. It has also been reported that 22-77% of patients with IgA nephropathy have IgA anti-gliadin antibodies and 3-4% of these have CD.8,9 It is curious that our patient has an MN without IgA deposits, the co-existence of which with CD is exceptional. We have only come across one case similar to ours,10 which showed a poor response to treatment that involved blocking the renin-angiotensin system combined with a gluten-free diet, contrary to what we observed in our patient. In another publication a link between CD and MN is described, but the patient was initially diagnosed with MN and, then, 10 years later, with CD, the opposite to what happened in the case we present.11 In our opinion, the link between CD and MN is not coincidental and we think they share an autoimmune pathogenesis. Firstly, both entities are simultaneous in their onset and it may be the case that the autoimmune changes facilitate both the digestive and renal symptoms. Secondly, the remission of the proteinuria coincides with the disappearance of the IgA anti-tissue transglutaminase antibodies; although the remission may have been spontaneous, the fact that they coincided at the same time means that we can establish some kind of link, as the majority of cases of MN remit when the original cause is addressed. We would like to emphasize that our patient has not received immunosuppressive treatment, as we regarded her MN as a secondary condition and, in addition, she had never had a full nephrotic syndrome.
We conclude that the appearance of proteinuria in patients with CD may be due to MN and that a gluten-free diet improves both entities, supporting the view that there are pathogenic mechanisms which are common to both diseases.
Figure 1.