Presentamos dos casos de infección por Strongyloides stercoralis (S. stercoralis) en pacientes trasplantados renales en nuestro centro. Se describen las características de su presentación clínica, el tratamiento y la resolución del mismo.
INTRODUCTION
Parasitic infections in transplant patients are infrequent.1 Of the 342 parasite species that can infect humans, only 5% have been described in transplant patients.2 In a series of 675 transplant patients, the prevalence of symptomatic parasite infections was 2.4%; S. stercoralis, followed by Giardia lamblia and Toxoplasma gondii were the most frequently implicated parasites.3 Up until now, Spanish literature has described multiple cases of S. stercoralis infection in the general population, but never in transplant patients.
Strongyloides stercoralis is an intestinal parasite which is endemic in some parts of Spain. Its infection can provoke isolated eosinophilia or even diarrhoea associated with haemoptysis and bronchospasm with a high mortality rate. In transplant patients, the symptoms can worsen with an increase in the number of adult worms, provoking multiple organ failure; in these patients, diagnosing the infection may be complicated. Clinical examination may sometimes mistake the symptoms for side effects from the drugs that are widely used in transplants, and for this reason, there must be a high suspicion index in order for it to be diagnosed correctly. We present two cases of active infection with S. stercoralis in kidney transplant patients in our centre, with their diagnostic procedure and evolution with treatment.
CASE 1
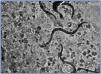
Male patient, aged 55 years in a chronic CAPD due to renal failure secondary to chronic glomerulonephritis. He received a kidney transplant from a cadaver donor in July 2005 and the immunosuppressants tacrolimus, mycophenolate mofetil and prednisone, and maintained a stable renal function with creatinine levels around 2.5mg/dl. Two years after the transplant, he presented a profile of persistent diarrhoea with 4-5 bowel movements a day and weight loss of 2kg. Microbiological analysis of the faeces detected numerous S. stercoralis larvae (figure 1). The eosinophil count was normal (0.3 x 103/μl). The patient resided in a part of the Valencian Community that is considered to be an endemic area for S. stercoralis. We reviewed the case history prior to the transplant, which revealed outbreaks of urticaria as well as mild asthma, which had been treated sporadically with bronchodilators. Pre-transplant analytical tests showed that the patient had intermittent mild eosinophilia (1.0 x 103/μl). Treatment was begun with thiabendazole in doses of 1.5g/12 hours (25mg/kg/day) administered orally during five days; the cycle was repeated after seven days due to persistence of the infection. 14 days after beginning treatment, the diarrhoea had not subsided, weight loss had reached 4kg and renal function had decreased. Consequently, the patient was admitted to receive intravenous hydration. Both the stool sample obtained during that stay and the one obtained following the second treatment with thiabendazole tested negative for parasites. The diarrhoea improved and the renal function recovered progressively until reaching normal creatinine levels. An additional stool sample was analysed for parasites one month later and tested negative. At present, the patient remains asymptomatic, the episodes of urticaria and asthma have improved, and renal function is stable. A study of family members residing in the same household detected S. stercoralis in the patient¿s son, who was treated with thiabendazole as an outpatient.
CASE 2
Male patient 51 years of age with renal failure secondary to Alport syndrome, on haemodialysis since 2003. The patient received a renal transplant from a cadaver donor in August 2006 and tacrolimus, mycophenolate mofetil and prednisone as immunosuppressants. The patient maintains a stable renal function with creatinine levels around 2mg/dl. Immediately following the transplant, the patient presented mild diarrhoea with 4-5 soft bowel movements daily, which were attributed to the treatment with mycophenolate mofetil. The patient also presented a disproportionate level of normocyticnormochromic anaemia for his level of renal failure, and that condition did not respond to treatment with erythropoietic agents. Seven months after the transplant, a routine analytical test showed an absolute eosinophilia of 3.7 x 103/μl that was confirmed with a second haematological sample. The patient continued to present the same clinical profile of 4-5 soft bowel movements as had been the case since the transplant, with no urticaria or asthma. Analysis of the stool showed numerous S. stercoralis larvae. Treatment was begun with albendazole in oral doses of 400mg every 12 hours during three weeks. After finishing the first treatment cycle, the patient presented a more normal daily number of bowel movements, had improved the eosinophil count to 0.8 x 103/μl and had normal haemoglobin levels. After this first cycle of abendazole treatment, the stool sample tested negative for S. stercoralis. We subsequently administered two new cycles of one week of treatment at one month and at two months after the diagnosis, and reached a completely normal peripheral eosinophil count. In the study of parasites in family members living in the same household, we identified signs compatible with Blastocystis hominis and Endolimax nana in the patient¿s wife, who was treated with oral metronidazole. Both reside in an area that is endemic for S. stercoralis.
DISCUSSION
S. stercoralis is an intestinal parasite that is particularly endemic in some parts of Spain, particularly along the Mediterranean coast and in populations close to the city of Valencia.4 Infection with S. stercoralis occurs when the patient¿s skin comes into contact with materials contaminated with human faeces; the larva then develops through its life cycle within the body, and becomes an adult worm that infects the digestive tract. Its larvae are excreted once again in the patient¿s stool. One aspect that makes this parasite particularly virulent is that larvae in the intestine may also mature and cross the colonic mucosa, which leads to chronic infection or re-infection.5
In immunocompetent patients, this auto-infection process can perpetuate without causing any symptoms other than sporadic eosinophilia, cases of diarrhoea or mild asthma, but in immunocompromised patients, there may be a massive proliferation of larvae throughout the digestive tract, lungs, liver, heart and central nervous system. This can lead to a multiple organ failure with a high mortality rate, known as hyperinfection syndrome.2 In transplant patients, infection with the parasite may become evident due to the immunosuppressant treatment. Normally, these are latent infections that were present before the transplant, but patients may also suffer a primary infection following the transplant, particularly those living in endemic areas.
Diagnosing S. stercoralis may be difficult. Eosinophilia may be the only anomaly, and it may not be present in transplant patients. For this reason, when a transplant patient presents a profile of diarrhoea that is not self-limited, we should rule out possible complications induced by drugs such as mycophenolate mofetil, especially if it is associated with the pro-kinetic effect of tacrolimus. Furthermore, if a conventional stool analysis gives a negative result, we should contemplate the possibility of diarrhoea secondary to a parasitic infection. When diagnosing parasitosis by S. stercoralis, it may be necessary to collect various fresh stool samples before detecting any larvae6 (figure 1) because a single sample does not show the infection in up to 70% of all cases. Detection sensitivity increases to 50% for direct vision of fresh stool when three samples are used, and to 100% with seven samples.7 If the stool samples are cultivated in an agar plate, as we did in these two cases, the infective larvae can be seen (figure 2). We also had a specific ELISA test with high sensitivity and specificity,6 and an indirect ELISA test for detecting the presence of IgG and IgM antibodies for S. stercoralis.8 In immunocompromised patients, the specific ELISA test could be indicated when there is a high suspicion of parasite infection and the stool samples give a repeated negative result; it could be helpful in achieving early diagnosis and treatment. Specific IgG antibodies may remain positive years after completing anthelmintic treatment.9
The treatment indicated for Strongyloidiasis is thiabendazole dosed at 25mg/kg/day, distributed in two doses. Two days of treatment are recommended for non-complicated infections, and five for complicated infections. In immunodepressed patients, repeating the cycle of thiabendazole is also recommended,10 as we did in the first case. The most common side effect is digestive intolerance. Albendazole in doses of 400mg/12 hours during three weeks, as we used in case 2, is a treatment alternative to thiabendazole that has also been proven effective against S. stercoralis, although clinical experience with the drug is more limited.11 Ivermectine with a dosage of 200mg/kg/day, distributed in two doses, is also an approved treatment and has fewer adverse effects than thiabendazole, but its prescription in Spain requires a foreign medication request. The effectiveness of the treatment must be documented with a negative stool sample upon completion of that treatment, and a new sample must be collected if the disease reappears; relapses are frequent. It has been indicated that cyclosporin could have an anti-parasitic effect against S. stercoralis in both mice12 and humans,13 and we must point out that neither of our patients received that particular immunosuppressant.
In conclusion, although infection with S. stercoralis is not very frequent, it can cause serious problems for a transplant patient if it is not diagnosed and treated in time. When evaluating candidates for receiving a kidney transplant, special attention must be paid to the presence of unexplained eosinophilia and a history of corticoidresistant pruritus or asthma in order to diagnose a possible parasitic infection and eradicate it before beginning treatment with immunosuppressants. Furthermore, differential diagnosis of diarrhoeal syndromes in kidney transplant patients must include testing fresh stool samples for possible parasites, especially if there is a clinical or analytical suspicion of S. stercoralis infection. If this infection is diagnosed, family members living in the same household should also be examined. The risk of relapse is frequent with this disease, which is why it is recommended that we always obtain a negative stool sample after completing the treatment and repeat the examination if there is a suspicion of a new S. stercolaris infection.
Figure 1.
Figure 2.