Patients on renal replacement therapy (RRT) infected with the human immunodeficiency virus (HIV) are a special group with growing interest. In order to study the epidemiological data of HIV+ patients on RRT in Spain, we collected individual information from 2004 to 2011 (period of use of highly active antiretroviral therapy [HAART]) in the Autonomous Communities of Andalusia, Aragon, Asturias, Catalonia, Valencia, Castilla la Mancha, Castilla León, Galicia, Madrid, La Rioja and the Basque Country, comprising 85% of the Spanish population. A total of 271 incident and 209 prevalent patients were analysed. They were compared with the remaining patients on RRT during the same period. The annual incidence was 0.8 patients per one million inhabitants, with a significant increase during the follow-up period. The proportion of prevalent HIV+ patients was 5.1 per 1000 patients on RRT (95% confidence interval [CI] 4.4–5.8). Although glomerular diseases constituted the majority of cases (42%), diabetic nephropathy was the cause in 14% of patients. The nation-wide totals for these percentages were 13 and 25%, respectively. Compared to the total of patients in treatment, the risk of death was significantly higher in the HIV+ group: hazard ratio (HR) adjusted for age, sex and diabetes was 2.26 (95%CI 1.74–2.91). Hepatitis C coinfection increased the risk of death in the HIV+ group (HR 1.77; 95%CI 1.10–2.85). The probability of kidney transplantation in HIV+ was only 17% after 7 years, comparing with total RTT patients (HR 0.15; 95%CI: 0.10–0.24).
Despite the use of HAART, the incidence of HIV+ patients on dialysis has increased; their mortality still exceeds non-HIV patients, and they have a very low rate of transplantation. It is necessary to further our knowledge of this disease in order to improve results.
Los pacientes con infección por el virus de la inmunodeficiencia humana (VIH) y enfermedad renal que terminan en tratamiento sustitutivo renal constituyen un grupo especial con interés creciente para la nefrología. Con el objetivo de conocer datos epidemiológicos de los pacientes VHI+ en España, recogimos información individualizada durante los años 2004 a 2011 (periodo de uso de tratamiento antiviral de alta eficacia) en las comunidades autónomas (CCAA) de Andalucía, Aragón, Asturias, Cataluña, Comunidad Valenciana, Castilla-La Mancha, Castilla y León, Galicia, Madrid, La Rioja y País Vasco, que comprendían un 85% de la población española. Se analizó a un total de 271 pacientes incidentes y 209 prevalentes. Se compararon con el resto de pacientes en tratamiento sustitutivo durante el mismo periodo de tiempo. La incidencia anual fue de 0,8 pacientes por millón de habitantes, con un aumento significativo a lo largo del periodo de seguimiento. La proporción de pacientes prevalentes VIH+ fue de 5,1/1.000 pacientes en tratamiento sustitutivo, intervalo de confianza (IC) del 95%: 4,4-5,8. Las causas glomerulares constituyeron la mayoría (42%), aunque hubo un 14% de nefropatía diabética. En el total de España, esos porcentajes son 13 y 25%, respectivamente. Comparando frente al total de pacientes en tratamiento, el riesgo de muerte fue significativamente mayor en el grupo VIH+: hazard ratio (HR) ajustado por edad, sexo y presencia de diabetes: 2,26 (IC 95%: 1,74-2,91). La coinfección por hepatitis C aumentó el riesgo de muerte dentro del grupo VIH+: HR 1,77 (IC 95%: 1,10-2,85). La probabilidad de recibir trasplante renal en los VIH+ solo alcanzó el 17% a los 7 años, comparando con el total de pacientes en diálisis HR: 0,15 (IC 95%: 0,10-0,24).
A pesar del uso de las nuevas combinaciones de antivirales, la incidencia de pacientes VIH+ en diálisis se ha incrementado, su mortalidad supera todavía al resto de pacientes, y tienen una tasa de trasplante muy baja. Se hace necesario profundizar en el conocimiento de esta enfermedad para mejorar los resultados.
The profile of patients with human immunodeficiency virus (HIV) infection has changed significantly since the mid-1990s, following the introduction of highly active anti-retroviral treatment (HAART). Whilst the majority of patients who started dialysis in the pre-HAART era did so because of HIV-associated nephropathy (HIVAN), in recent years the causes of renal disease in these patients have become more diverse. In addition to HIVAN, the main causes of end stage renal disease include diabetic nephropathy, nephrosclerosis, and nephrotoxicity.1 However, the overall incidence of chronic kidney disease requiring renal replacement therapy (CKD-5D) in these patients has not decreased.
Patients with HIV infection differ from other renal patient groups in certain aspects.2 Level of immunocompetence determines patient selection for the transplant waiting list, and, to achieve an acceptable survival rate, a special protocol is needed for post-transplant management.3 However, despite the use of specific protocols, a high rate of delayed graft function has been observed in patients with HIV.4 In addition, the co-existence of renal failure and HIV infection increases the risk of cardiovascular disease.5
In Spain, the incidence and prevalence of HIV infection in patients with CKD-5D are thought to be low, but there are no or global studies that quantify these rates.6
There are several publications describing the specific characteristics of such Spanish patients. This reflects an existing interest in better understanding this problem.7 Recently, 3 scientific societies (Sociedad Española de Nefrología [Spanish Society of Nephrology], Grupo de Estudio del SIDA-SEIMC [AIDS Task Force and Spanish Society of Infectious Diseases], and Sociedad Española de Química Clínica y Enfermedad Molecular [Spanish Society of Clinical Chemistry and Molecular Disease]) produced an exhaustive consensus document on the assessment and management of renal disease in patients with HIV infection. This document contains specific recommendations for patients in the various stages of kidney disease, also on dialysis, and for transplanted patients. It is available on the websites of the 3 participating scientific societies.8
Currently, almost all the regions in Spain have a registry of patients with CKD-5D. In addition to basic information, these registries contain data on viral infections such as hepatitis B, hepatitis C, and HIV.
In light of the above, we proposed an epidemiological study in Spain, based on the existing registries. The aim was to describe the causes of primary renal disease, the incidence and prevalence of HIV infection in dialysis and transplant patients, survival, and the factors that can influence survival.
Patients and methodsWe requested information from the renal patient registries of the following regions (so called autonomous communities) in Spain: Andalusia, Aragón, Asturias, Catalonia, Valencian Community, Castile-La Mancha, Castile and León, Galicia, Madrid, La Rioja, and Basque Country. This group of communities encompasses 85% of the total Spanish population. Information was collected on patients on renal replacement therapy with HIV-positive serology. Incident patients were those starting renal replacement therapy between 1 January 2004 and 31 December 2011, and prevalent patients were those on renal replacement therapy at 31 December 2011. The following variables were collected: date of birth; sex; date and modality of first replacement therapy and subsequent treatments; aetiology of primary kidney disease, coded according to the European Renal Association-European Dialysis and Transplantation Association (ERA-EDTA) 1995 coding system; hepatitis B and hepatitis C serological status upon starting treatment; date of death if applicable; and the autonomous community where the patient was registered. The data from the various autonomous communities were grouped together in one single file.
For the comparison group, we used a database of all RRT incident patients between the years 2004 and 2011 from the same group of autonomous communities (with the exception of Madrid and La Rioja because their registries did not include complete information for the same period of time). This database did not contain information on HIV status. However, given the low incidence and prevalence of HIV in general, the risk estimation error in considering the whole group as unexposed to HIV was negligible. This database comprised 30693 patients. The results of survival analysis were presented at the registries meeting of the Sociedad Española de Nefrología (SEN) 2013 conference.9
To calculate incidence, the population of each autonomous community was taken at the mid-point of the 8-year interval, onJanuary first 2008, from the 2001 census estimated population. To calculate prevalence, the population at 31 December 2011 was used. The data were obtained from the Instituto Nacional de Estadística (National Institute of Statistics) (www.ine.es).
Statistical analysisDescriptive statistics were used, such as mean, median, standard deviation, and percentage frequency. For comparison of frequencies, a standard chi-square test was used; when this was not appropriate, Fisher's exact test was used. The comparison of means between 2 groups was performed using the t-test (based on Student's t-distribution). For adjusted comparison of the proportion of diabetic nephropathy in the group with HIV vs the whole group, the Mantel–Haenszel method was used, stratified by age.
For survival analysis, only the database of RRT incident patients was used. The Kaplan Meier method was used for unadjusted analysis and Cox regression was used for adjusted analysis of survival. The adjustment variables used were age (as a continuous linear variable), sex, and diabetes as the cause of primary kidney disease. Diabetes was used because in different presentations of the Dialysis and Transplant Registry it was observed that this variable had great prognostic significance.9
In calculating the probability of transplantation over the study period, the group of incident patients with HIV was compared with the all patients on dialysis. The competing risk between death and transplantation was taken into account, because deceased patients would not have the option of transplant, nor could they be considered lost to follow-up. The Fine and Gray model, as applied by Scrucca,10 was used for the R project programme. In this model, the adjustment variables used were age, as a continuous variable without transformation, and sex. To test what variables were associated with changes in incidence, Poisson regression was used. The statistical calculations were performed using the software R project version 2.13, with GNU licence.11
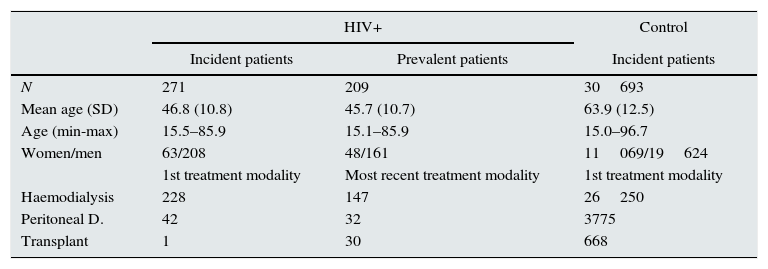
ResultsIncidence, prevalence, and primary renal diseaseA total of 271 incident patients and 209 prevalent patients were selected. The demographic data of both groups and the first replacement therapy modality used are shown in Table 1.
Demographic data of incident and prevalent patients with HIV against total control population started on renal replacement therapy.
| HIV+ | Control | ||
|---|---|---|---|
| Incident patients | Prevalent patients | Incident patients | |
| N | 271 | 209 | 30693 |
| Mean age (SD) | 46.8 (10.8) | 45.7 (10.7) | 63.9 (12.5) |
| Age (min-max) | 15.5–85.9 | 15.1–85.9 | 15.0–96.7 |
| Women/men | 63/208 | 48/161 | 11069/19624 |
| 1st treatment modality | Most recent treatment modality | 1st treatment modality | |
| Haemodialysis | 228 | 147 | 26250 |
| Peritoneal D. | 42 | 32 | 3775 |
| Transplant | 1 | 30 | 668 |
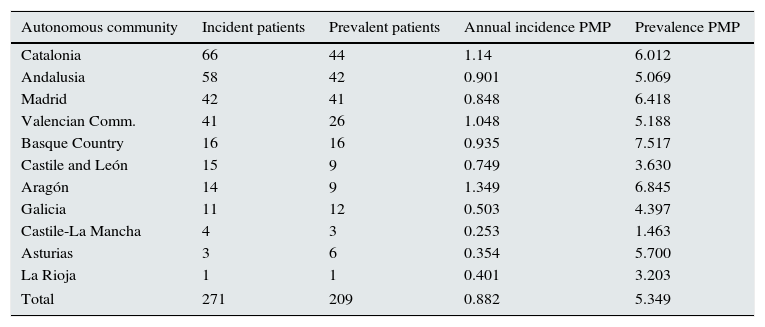
The results of annual incidence at the mid-point of the 8-year period, and the prevalence at 31 December 2011, grouped by participating community, are presented in Table 2; rates are expressed as number of patients per million population. The prevalence of patients with HIV was 5.1 per 1000 patients on replacement therapy; 95% confidence interval (95%CI), 4.4–5.8 per 1000 patients on replacement therapy.
Number of patients per autonomous community, with calculations of incidence and prevalence per million population.
| Autonomous community | Incident patients | Prevalent patients | Annual incidence PMP | Prevalence PMP |
|---|---|---|---|---|
| Catalonia | 66 | 44 | 1.14 | 6.012 |
| Andalusia | 58 | 42 | 0.901 | 5.069 |
| Madrid | 42 | 41 | 0.848 | 6.418 |
| Valencian Comm. | 41 | 26 | 1.048 | 5.188 |
| Basque Country | 16 | 16 | 0.935 | 7.517 |
| Castile and León | 15 | 9 | 0.749 | 3.630 |
| Aragón | 14 | 9 | 1.349 | 6.845 |
| Galicia | 11 | 12 | 0.503 | 4.397 |
| Castile-La Mancha | 4 | 3 | 0.253 | 1.463 |
| Asturias | 3 | 6 | 0.354 | 5.700 |
| La Rioja | 1 | 1 | 0.401 | 3.203 |
| Total | 271 | 209 | 0.882 | 5.349 |
Using the general data on prevalent and incident patients from the registries of the participating communities, the proportion of incident patients with HIV over the total incident patients was calculated at 0.74%. In prevalent patients, this proportion was 0.51%.
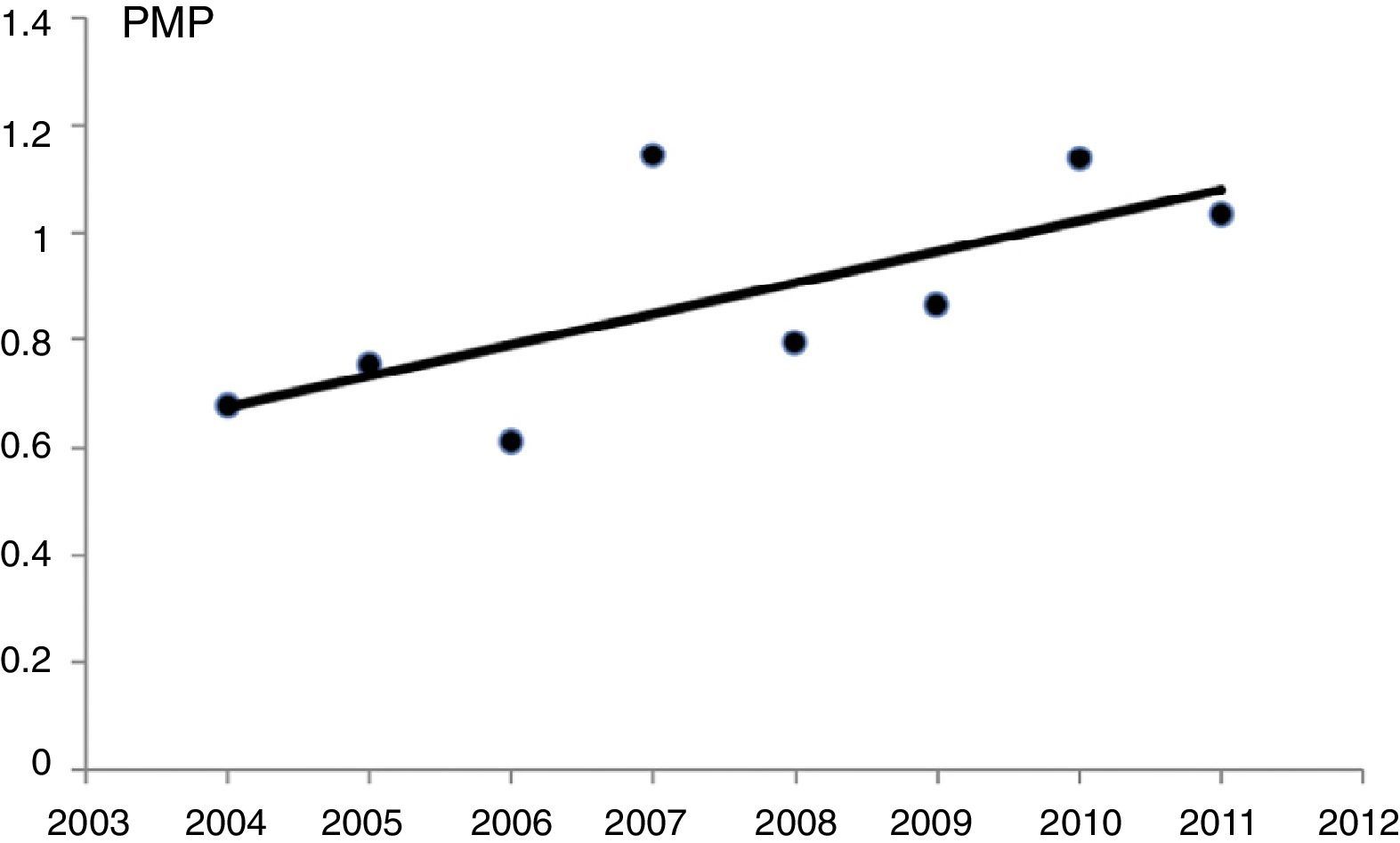
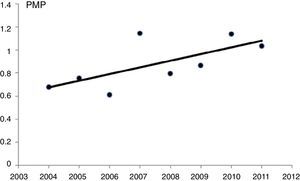
Poisson regression, without adjustment variables, showed a statistically significant increase in the incidence of patients with on renal replacement therapy from the year 2004 to 2011 (P=.014). With this method, there was an estimated annual increase of 6.8% (95%CI, 1.3%–12.5%) (Fig. 1).
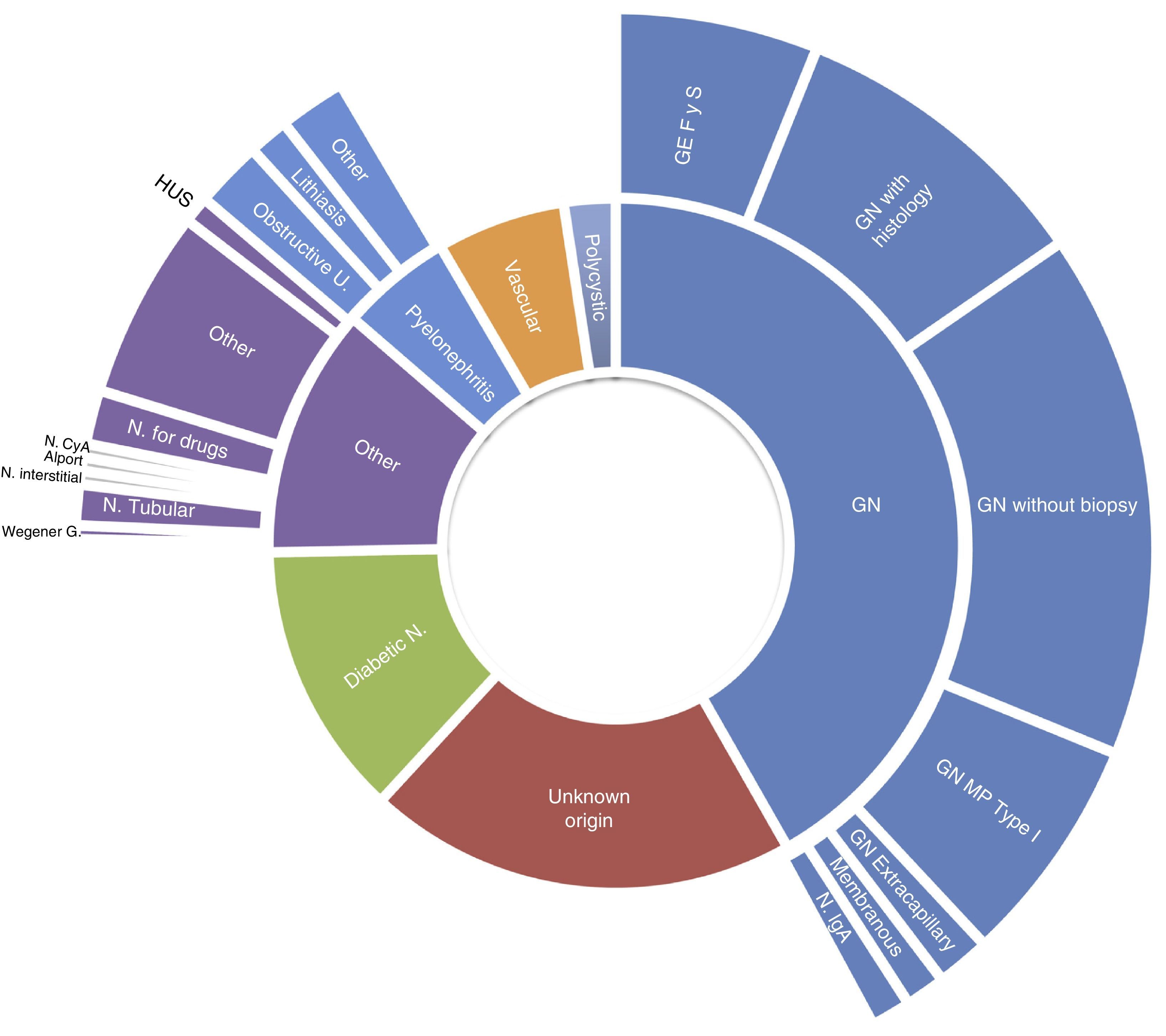
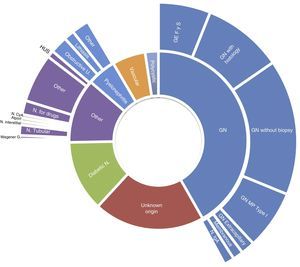
The frequency of causes of primary renal disease in incident patients grouped by EDTA registry classification, was as follows: glomerular, 42%; unknown aetiology, 20%; diabetic nephropathy, 13%; other, 12%; chronic pyelonephritis, 5%; vascular/hypertensive nephropathy, 5%; polycystic, 2%; and ischaemic nephropathy, 1%. More detailed causes are shown in Fig. 2. For the total patients on renal replacement therapy in the same communities, diabetic nephropathy was the cause in 22% of incident patients, and glomerular causes affected 12%. In the comparison of the percentage of incident patients with diabetic nephropathy, between the group with HIV and those without, adjusting for age category, the rate ratio using Mantel–Haenszel was 0.50 (95%CI, 0.35–0.71).
There were no significant changes in the incidence of each diagnostic group of primary renal disease in the different years of follow-up, nor were there differences in aetiology when comparing different periods before and after 2 cut-off points: January first 2008 and January first 2006.
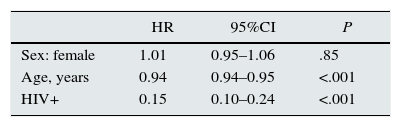
Renal transplantDuring the period observed, of the 271 incident patients with HIV, 21 patients received at least one renal transplant, one of which was for the second time. The cumulative probability of receiving a first renal transplant during the period observed, taking into account the competing risks of death and transplantation, was 5%, 10%, and 17%, at 2 years, 5 years, and 7 years, respectively, vs 19%, 26%, and 32%, for the total patients on replacement therapy. These are gross comparisons without adjustment for confounding variables.
The risk ratio for receiving a renal transplant for patients with HIV compared with patients without HIV was estimated using the Fine and Gray competing risks regression model, adjusted for age and sex. This estimation gave a value of 0.15 (95%CI, 0.10–0.14). This means that the probability of receiving a renal transplant during the time studied was estimated to be 6.6 times lower for patients with HIV than for patients without HIV, 95%CI, 4.2–10 times (Table 3).
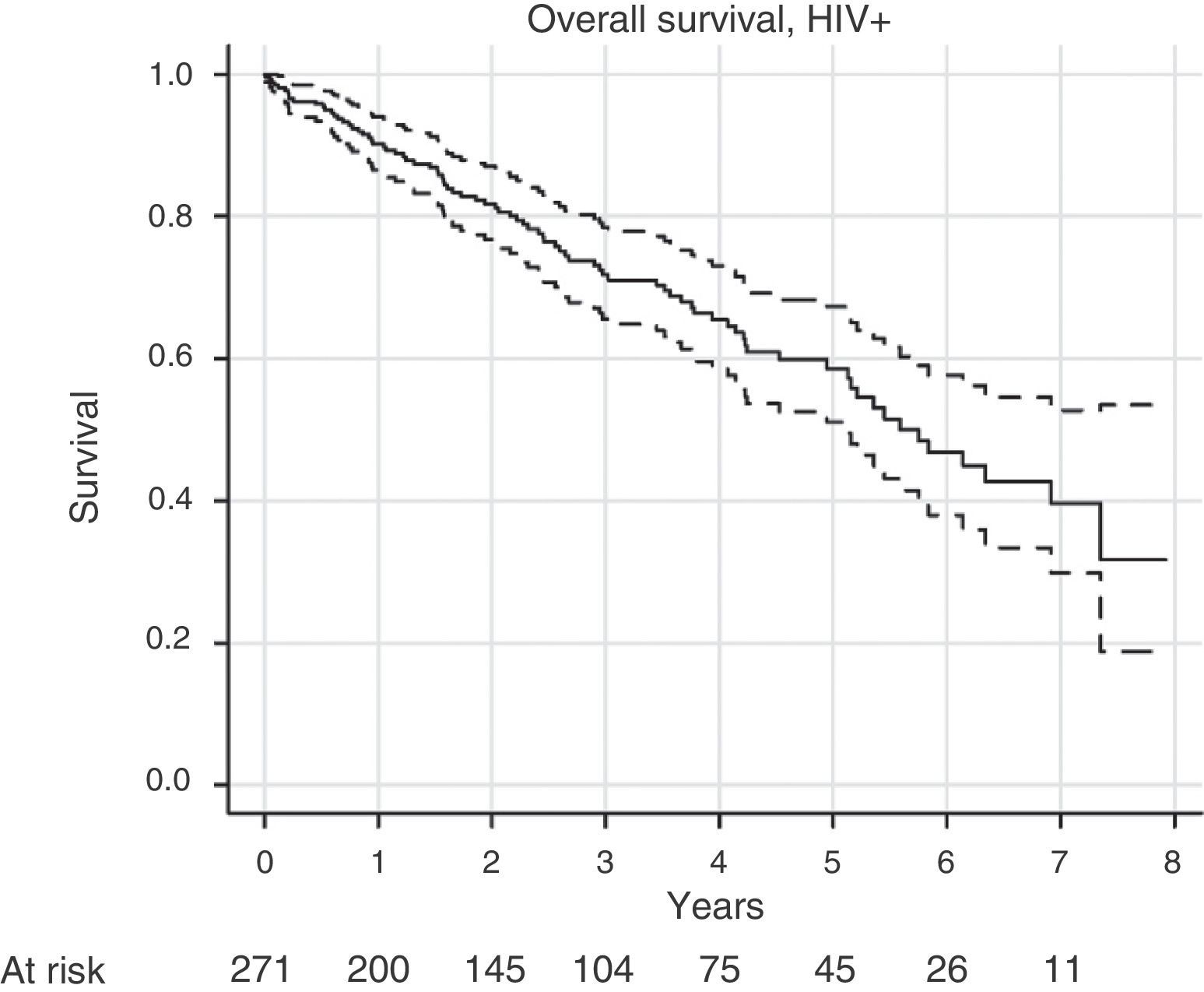
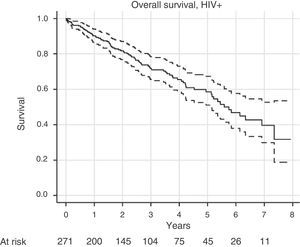
SurvivalFor survival analysis, we used the cohort of RRT incident patients with HIV. Of the total 271 patients in this group, 84 patients died. The median survival, estimated using the Kaplan–Meier method, was 5.75 years (95%CI, 5.13–7.35 years). The survival graph is shown in Fig. 3.
Using the Cox model, we investigated variables that could be associated with survival. The variables investigated were age, sex, diabetic nephropathy, and period when treatment was started: either before or after January 2008 (to test if survival improved over time). None were found to be statistically significant.
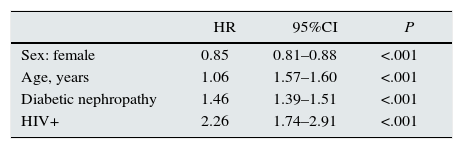
Survival was compared between incident patients with HIV and all incident patients from the same group of communities between the years 2004 and 2011. Cox regression analysis, adjusted for age, sex, and diabetic nephropathy, estimated a mortality risk for patients with HIV of just over 2 times higher than for patients without HIV; HR, 2.26 (95%CI, 1.74–2.91) (Table 4).
Cox regression model for overall survival, with the variables indicated.
| HR | 95%CI | P | |
|---|---|---|---|
| Sex: female | 0.85 | 0.81–0.88 | <.001 |
| Age, years | 1.06 | 1.57–1.60 | <.001 |
| Diabetic nephropathy | 1.46 | 1.39–1.51 | <.001 |
| HIV+ | 2.26 | 1.74–2.91 | <.001 |
Estimated effect of having HIV+ serology compared with HIV− serology, adjusting for age, sex, and diabetic nephropathy.
Of the patients with HIV, 7.8% had co-infection with hepatitis B (HBsAg+), and 53.1% had anti-hepatitis C antibodies. Given the low proportion of patients with hepatitis B, no exploratory analysis was performed for this group. The group that was seropositive for hepatitis C (HCV) was significantly younger, mean age 45.5 years vs 48.4 years (P=.034). There were no significant differences for sex, year of starting RRT, or incidence of diabetic nephropathy.
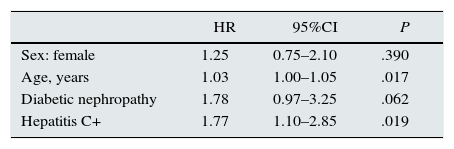
Using Cox regression, survival in the group of incident patients with HIV was analysed according to HCV-positive serology, adjusted for age, sex, and diabetic nephropathy. The risk of death for patients with HCV coinfection was almost 2 times higher than for patients with HIV monoinfection, HR, 1.77 (95%CI, 1.10–2.85) (Table 5).
Cox regression model for overall survival in the cohort of patients with HIV, estimating the effect of having hepatitis C positive serology, as well as the effects of age, diabetic nephropathy, and sex.
| HR | 95%CI | P | |
|---|---|---|---|
| Sex: female | 1.25 | 0.75–2.10 | .390 |
| Age, years | 1.03 | 1.00–1.05 | .017 |
| Diabetic nephropathy | 1.78 | 0.97–3.25 | .062 |
| Hepatitis C+ | 1.77 | 1.10–2.85 | .019 |
In Spain, the incidence of HIV infection in patients with CKD-5D during the period 2004–2011 was almost 1%. This is similar to that seen in other European series. The prevalence was lower than this. The prevalence, lower than the incidence, could be due to a worse survival of this patient group compared to other patients. We found a progressive increase in the incidence of HIV, which could not be attributed to an increase in HIV infection in the general population, as this has decreased since 1998. It could be related to an older patient age at time of HIV diagnosis and the increased survival since the use of HAART. This confers an increased exposure to risk factors for renal disease, such as toxicity from antivirals, co-infection with HCV, hypertension, dyslipidaemia, and diabetes.12 There is a great deal of evidence that patients with HIV infection, independently of sex or age, have a higher risk of developing atherosclerotic cardiovascular disease.13 HIV is associated with, and constitutes a risk factor for, chronic kidney disease.
Regarding the proportion of RRT prevalent patients with HIV in 2011 over patients without HIV on RRT, our results were very similar to those published by the Spanish group GESIDA in a survey carried out in 2006,6 and the confidence intervals overlapped. Five years elapsed between the estimation of these rates, and we detected an annual increase in the incidence of HIV. However, it is possible that the progressive increase in the prevalence of total CKD-5D patients and their improved survival compensated for the increase in incident patients with HIV.
If we take the prevalence of HIV infection in the adult population to be 1.5/1000, as was published for the year 2008 in Andalusia,14 then using our data and the data from the dialysis and transplant registries, we can estimate the prevalence ratio of renal replacement therapy in those infected with HIV in the general population to be 3.5. This ratio, under certain conditions,15 can be interpreted as the relative risk of developing CKD-5D for those infected with HIV vs the general population.
These results also show that infection with HIV in CKD-5D confers an increased mortality risk. It is important to put these findings into context: the epidemiological data from Catalonia during the era of extensive HAART use reported a mortality for patients with HIV 5 times higher than that of the general population.16 In the last 2 decades, the prognosis of patients with HIV has improved substantially. This improvement was further reinforced once sufficient experience was acquired for management of the pharmacokinetics of HAART on dialysis.17 It has been reported that this improved survival now equals that of patients without HIV: one study conducted in France concluded that HIV infection did not increase the risk of death in patients on dialysis or with a transplant.18 However, the analysis of a cohort of patients with HIV that started dialysis in Spain during the years 1999–2006 observed that their survival was lower than that of other patients starting dialysis.19 Our results are in line with the second observation, probably because the patients had the same demographic origin and similar risk factors.
On top of the already increased risk with HIV, co-infection with HCV is associated with yet higher mortality, up to nearly double the risk of death by any cause. It has been reported that coinfection with HCV is associated with HIV infection through the intravenous use of illegal drugs, and a longer duration of HIV infection.6 These 2 factors could lead to a worse survival.
The prevalence of HCV in our cohort was double that reported in Europe2 and was similar to that of other studies in Spain. This difference could be explained by a higher proportion of HIV infection via intravenous drug use in Spain. In one meta-analysis that included a total of 145608 patients on dialysis, HCV-positive serology was associated with a higher mortality in CKD-5D patients.20 Likewise, in patients with HIV infection without renal disease, HCV coinfection carries a higher mortality risk.21
The causes of primary renal disease in patients with HIV differed from those without HIV when compared with the dialysis and transplant registry of 2010. There was a notable predominance of glomerular causes: one third of patients with HIV vs 21% in the general registry. In the group with HIV, focal segmental glomerulosclerosis was a predominant cause, and probably corresponded to HIV-associated nephropathy (HIVAN), given that a specific code for HIVAN does not appear on the EDTA list. Therefore, focal segmental glomerulosclerosis likely represents a much lower percentage than that reported in the American series, as would be expected in a country with a small black population.
Other glomerular causes remained predominant, probably due to the association between HCV coinfection and glomerulopathies.22
Although diabetic nephropathy was less common in the group with HIV than in the general registry, 13% vs 22%, it remained a significant reason for starting dialysis. A progressive decrease was demonstrated in the incidence of HIVAN between 1995 and 2004 during the implementation of HAART.23 Also, in a review of autopsies of patients with HIV on HAART, the diagnosis of nephrosclerosis was one of the most common.24 It appears that in the last 10 years in Spain, when the use of HAART became widespread, the spectrum of primary renal disease in patients with HIV has changed, with a trend towards diagnoses akin to those of the CKD-5D population without HIV. The lack of changes in diagnoses over time in our data could be because in the period studied, HAART was already implemented and there were no major changes in the treatment of HIV. Therefore, although HAART has managed to control the course of HIV infection, the incidence of CKD-5 has not decreased. Additional strategies are needed for the prevention of other causes of glomerulonephritis and HIVAN, such as diabetic nephropathy, vascular causes, and nephrotoxicity.
As anticipated, we observed a low rate of renal transplant in patients with HIV. Even adjusting for the competing risk between death and renal transplant, the probability of receiving a renal transplant was much lower in this group than in the rest of the patients on dialysis. It was striking that although HIV infection is not a contra-indication for renal transplant, patients with HIV are 7 times less likely to receive a renal transplant than patients without HIV.
Given the low number of transplants performed, just 21, survival analysis was not performed for this particular group due to the low statistical power for detecting differences.
In this study, there was a lack of detailed information including variables such as type and history of antiviral treatment, time with HIV infection, route of transmission, cause of death, and many others. Therefore, a more detailed analysis was not possible. However, as the study was based on patient registries, the information obtained was of a high quality, guaranteed by the established data validation procedures of the participating registries. Furthermore, we had access to all existing cases in the communities studied, which, due to their demographic characteristics and percentage of the population included, can be taken as representative of the Spanish population as a whole.
We think that this study could serve to increase the understanding of the characteristics of patients with HIV and CKD-5D in Spain and thus continue improving the care for this serious health problem.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Saracho R, Martín Escobar E, Comas Farnés J, Arcos E, Mazuecos Blanca A, Gentil Govantes MÁ, et al. Evolución clínica de los enfermos renales crónicos en tratamiento sustitutivo con infección por VIH. Nefrologia. 2015;35:457–464.