Fibroblast growth factor 23 (FGF-23) is a phosphorus-regulating hormone. In chronic kidney disease (CKD), circulating FGF-23 levels are markedly elevated and independently associated with mortality. Left ventricular hypertrophy (LVH) is a potent risk factor for mortality in CKD, and FGFs have been implicated in the pathogenesis of myocardial hypertrophy. In addition, the effect of anemia on CV disease and LVH is well known in CKD. A relation between iron and FGF-23 metabolism is mentioned in a few studies. The aim of this study was to test the association of FGF-23 levels with echocardiographic (ECHO) and iron parameters in peritoneal dialysis patients (PD).
MethodsIn this cross-sectional study, 61 subjects with PD (29 women and 32 men, mean age: 46.9±13.3 years, mean PD vintage: 69.5±39 months) underwent echocardiograms to assess left ventricular mass index (LVMI). Medical treatments and average values of the basic laboratory results of the last 6 months for all patients were recorded. Serum FGF-23 concentrations were measured using intact FGF-23 (iFGF-23) human enzyme-linked immunosorbent assay (ELİSA) kit. According to the median levels of serum FGF-23 the patients were grouped into two (FGF-23 high and low groups).
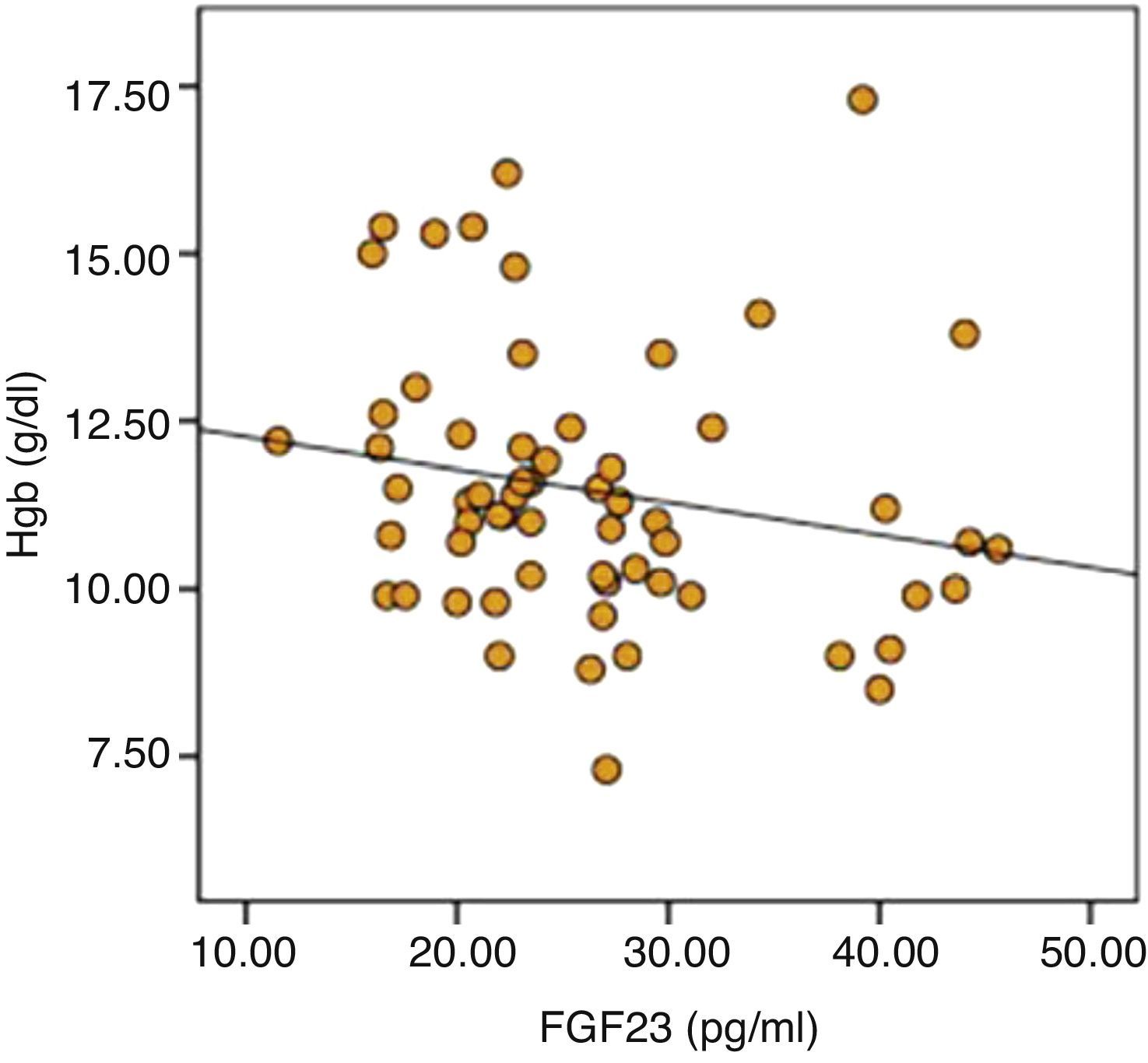
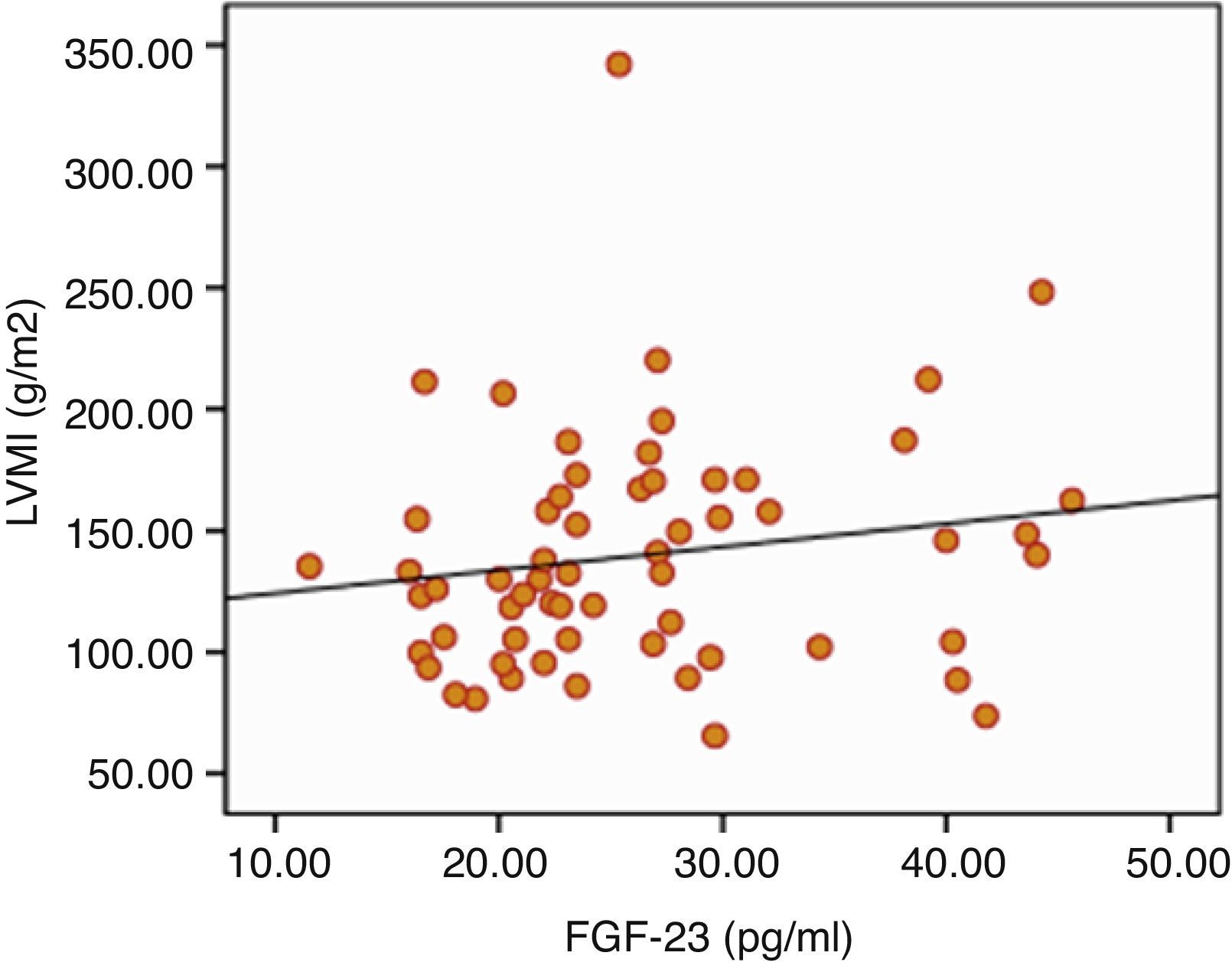
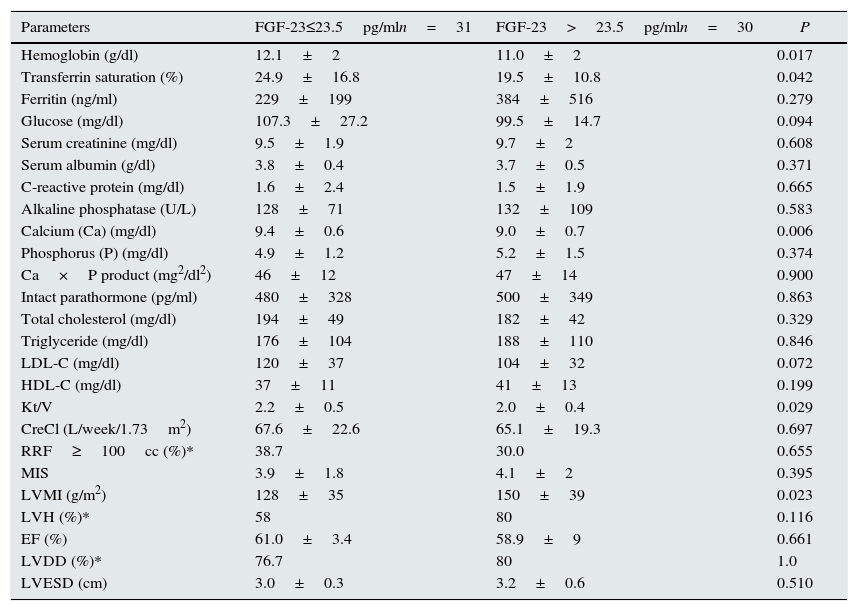
ResultsSignificant positive correlation was recorded between serum FGF-23 levels and LVMI (P=0.023). There was also significant difference in terms of hemoglobin (12.1±2 versus 11.0±2, P=0.017), transferrin saturation (TSAT) (24.9±16.8 versus 19.5±10.8, P=0.042) between low and high FGF-23 group. Also in linear regression analysis the negative relation between FGF-23 and hemoglobin is persisted (r=0.199, P=0.045).
ConclusionsFGF-23 is associated with LVMI, anemia and low TSAT in patients with PD. Whether increased FGF-23 is a marker or a potential mechanism of myocardial hypertrophy and anemia in patients with end-stage renal disease (ESRD) requires further study.
El factor de crecimiento fibroblástico 23 (FGF-23) es una hormona reguladora del fósforo. En la enfermedad renal crónica (ERC), los niveles de FGF-23 son especialmente elevados y se relacionan de manera independiente con mortalidad. La hipertrofia ventricular izquierda (HVI) es un importante factor de riesgo de mortalidad en la ERC y se ha implicado a los FGF en la patogenia de la hipertrofia del miocardio. Además, se conoce el efecto de la anemia en la enfermedad cardiovascular y la HVI en la ERC. En algunos estudios se menciona una relación entre el hierro y el metabolismo del FGF-23. El objetivo de este estudio fue comprobar la asociación de los niveles de FGF-23 con parámetros ecocardiográficos y de hierro en pacientes con diálisis peritoneal (DP).
MetodologíaEn este estudio transversal se procedió a realizar un ecocardiograma a 61individuos con DP (29 mujeres y 32 hombres; media de edad: 46,9±13,3 años; DP clásica media: 69,5±39 meses) para evaluar el índice de masa ventricular izquierda (IMVI). Se registraron los tratamientos médicos y los valores promedio de los resultados básicos de laboratorio de los últimos 6 meses de todos los pacientes. Las concentraciones en suero del FGF-23 se midieron con el kit ELISA (enzyme-linked immunosorbent assay) de FGF-23 humano intacto (iFGF-23). Según los niveles promedio de FGF-23 en suero, los pacientes se distribuyeron en dos grupos (FGF-23 alto y bajo).
ResultadosSe registró una correlación positiva significativa entre los niveles de FGF-23 en suero e IMVI (P=0,023). También hubo diferencias significativas en cuanto a la hemoglobina (12,1±2 frente a 11,0±2, P=0,017) y saturación de la transferrina (TSAT; 24,9±16,8 frente a 19,5±10,8, P=0,042) entre los grupos de FGF-23 bajo y alto. También en el análisis de regresión lineal se mantuvo la relación negativa entre el FGF-23 y la hemoglobina (r=0,199, P=0,045).
ConclusionesEl FGF-23 se asocia con IMVI, anemia y TSAT baja en pacientes con DP. Saber si el aumento del FGF-23 es un marcador o un mecanismo potencial de la hipertrofia miocárdica y la anemia en pacientes con insuficiencia renal terminal exige un estudio en mayor detalle.
Cardiovascular disease (CVD) is the most common cause of mortality in patients with end stage renal disease (ESRD)1. Besides the well-known traditional cardiovascular risk factors, this population has risk factors specific to chronic kidney disease which results in accelerated atherosclerosis2. LVH, commonly exists in patients with ESRD, is a major cardiovascular risk factor and independent predictor of cardiovascular mortality in this population3.
Hypertension is accepted as the main underlying factor in the development of LVH. However recent studies indicated that FGF-23 may contribute to left ventricular mass enlargement4,5. FGF-23 is phosphorus regulating hormone and significantly increased in patients with ESRD. It promotes phosphate excretion and reduces 1-25(OH)2 vitamin D production in the kidney6. Independent relation between increased FGF-23 levels and mortality was first reported by Guirez et al.7 in 2008 in hemodialysis (HD) patients. Even in patients with normal renal functions higher FGF-23 was found to be independently associated with mortality and cardiovascular events8. In addition, the effect of anemia on CV disease and LVH is well known in CKD patients9. A relation between iron and FGF-23 metabolism is mentioned in a few studies. In studies, it is emphasized that iron deficiency is an important stimulator of FGF-23 transcription10. The aim of this cross-sectional study was to investigate the possible relationship between serum levels of FGF-23 with ECHO and iron parameters in PD patients.
Materials and methodsSubjectsThis study was performed in peritoneal dialysis patients that are regularly followed in Ankara Diskapi Yildirim Beyazit Training and Research Hospital. Patients that suffered peritonitis or acute coronary event within last 2 months and patients with active malignancy were excluded. Sixty-one patients were included in the study. Fifty-one patients were on continuous ambulatory peritoneal dialysis program and 10 on automated peritoneal dialysis program. In all patients, the doses of dialysis were prescribed so as to generate a Kt/V of >1.7. Demographic characteristics, co-morbidities, medical treatments and average values of the basic laboratory results of the last 6 months for all patients were recorded. Residual renal function was expressed as the mean value of the residual urea and creatinine clearances. Peritoneal equilibration test (PET) was applied according to the method described by Twardowski et al.11.
FGF-23 measurementsIn all patients, 5ml of blood samples were obtained from peripheral vein after an overnight fasting into standard tubes biochemistry between 08.00 and 12.00AM. Within 30min of collection, samples were centrifuged at 2000rpm for 10min and stored in −80°C for maximum 1 month. Serum FGF-23 concentrations were measured using human iFGF-23 ELISA kit (Uscn Life Science Inc., Wuhan, China) which has typically a sensitivity of less than 4pg/ml.
EchocardiographyAll ECHO measurements were made according to the recommendations of the American Society of Echocardiography12. Patients were evaluated in semi-supine position in a dark room by the same cardiologist who was unaware of the clinical and laboratory data of the patients. Ejection fraction (EF), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), left ventricular posterior wall thickness (PWT), inter-ventricular wall thickness (IVWT) and left ventricular relaxation was evaluated in parasternal plane according to the guideline. The LVMI was calculated with the Devereux formula13. Body surface area was calculated with the formula of DuBois and DuBois14. Cut-off values for LVH were defined as 131g/m2 and 100g/m2 for men and women by considering the measurements in the Framingham Heart Study15,16.
EthicsThis study was approved by the local ethics committee of Ankara Diskapi Yildirim Beyazit Training and Research Hospital. The study was conducted in accordance with Declaration of Helsinki and all participants provided written informed consents.
Statistical analysisData analysis was performed by using “SPSS for Windows 19” (SPSS Inc., Chicago, USA). Results of the analysis of continuous variables were expressed as mean±SD and median [min–max] while the results of analysis of discrete variables were expressed as frequency distributions and percentages. Median serum FGF-23 value was used as the cut-off point and low-and high-FGF-23 groups were formed. In FGF-23 groups, demographic, clinical and laboratory data were compared pairwise. The Kolmogorov–Smirnov test was used for testing normality of continuous variables. Normally distributed variables were analyzed by t-test while others were analyzed with the Mann–Whitney U test. Chi-square test was used for comparison of discrete variables. Linear regression analysis model was used to study the relationship between LVMI and FGF-23. P<0.05 was considered statistically significant.
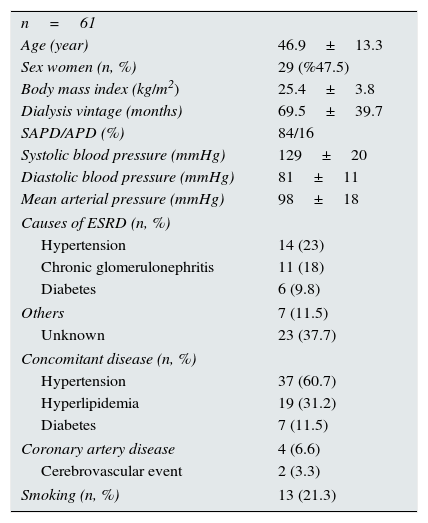
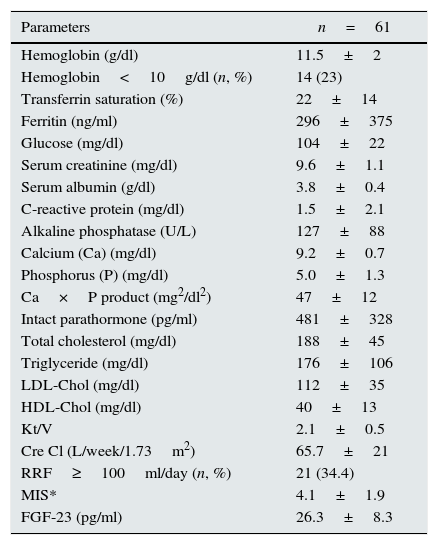
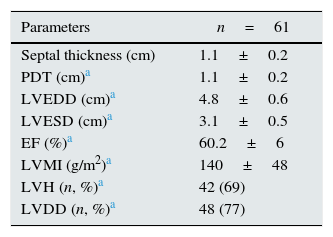
ResultsThere were 61 PD patients (32 men and 29 women, mean age 46.9±13.3 years) that fulfill the inclusion and exclusion criteria. Demographic characteristics, co-morbidities, medical treatments and basic laboratory results of the patients are presented in Tables 1 and 2. The average LVMI of the patients was 140±48g/m2 and LVH was present in 69% of patients according to the criteria presented in ‘Methods’ section. This prevalence was consistent with the previous studies17,18. Although the average EF of patients was 60.2±6%, left ventricular relaxation dysfunction (LVRD) was observed in majority of the patients (48 patients, 77%) (Table 4). Mean serum FGF-23 level was determined to be 26.3±8.3pg/ml [median 23.5pg/ml (min–max; 11.53–45.63)] (Table 3) and this value was similar to the values observed in previous studies19. Median value of FGF-23 (23.5pg/ml) was taken as a cut-off point and patients were divided into the low-and high-FGF-23 groups. Demographic, somatometric, laboratory and ECHO results of the two groups were compared (Table 5). No statistically significant difference was observed between the two groups with regard to age, gender, duration of dialysis, BMI, waist circumference, presence of co-morbid diseases, smoking, and medical treatments. There were statistically significant differences in systolic blood pressure (SBP) and mean blood pressure (MBP) between low and high FGF-23 groups (134.8±20.5 versus 126.3±16.5mmHg; P=0.04 and 96.3±17.6 versus 103±17.6mmHg; P=0.04 respectively). However no statistically significant difference was observed for DBP (73.8±10.9 versus 83.3±11.7, P=0.157). There was also significant difference in terms of hemoglobin (12.1±2 versus 11.0±2, P=0.017), TSAT (24.9±16.8 versus 19.5±10.8, P=0.042), albumin-corrected serum calcium (9.4±0.6 versus 9.0±0.7, P=0.006), and Kt/V (2.2±0.5 versus 2.0±0.4, P=0.029) between low and high FGF-23 groups.
Properties of patients.
| n=61 | |
| Age (year) | 46.9±13.3 |
| Sex women (n, %) | 29 (%47.5) |
| Body mass index (kg/m2) | 25.4±3.8 |
| Dialysis vintage (months) | 69.5±39.7 |
| SAPD/APD (%) | 84/16 |
| Systolic blood pressure (mmHg) | 129±20 |
| Diastolic blood pressure (mmHg) | 81±11 |
| Mean arterial pressure (mmHg) | 98±18 |
| Causes of ESRD (n, %) | |
| Hypertension | 14 (23) |
| Chronic glomerulonephritis | 11 (18) |
| Diabetes | 6 (9.8) |
| Others | 7 (11.5) |
| Unknown | 23 (37.7) |
| Concomitant disease (n, %) | |
| Hypertension | 37 (60.7) |
| Hyperlipidemia | 19 (31.2) |
| Diabetes | 7 (11.5) |
| Coronary artery disease | 4 (6.6) |
| Cerebrovascular event | 2 (3.3) |
| Smoking (n, %) | 13 (21.3) |
SAPD/APD, sustained ambulatory peritoneal dialysis/instrumental peritoneal dialysis; ESRD, end stage renal disease.
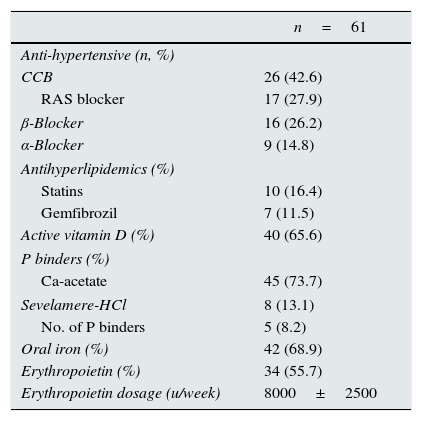
Treatment properties of patients.
| n=61 | |
|---|---|
| Anti-hypertensive (n, %) | |
| CCB | 26 (42.6) |
| RAS blocker | 17 (27.9) |
| β-Blocker | 16 (26.2) |
| α-Blocker | 9 (14.8) |
| Antihyperlipidemics (%) | |
| Statins | 10 (16.4) |
| Gemfibrozil | 7 (11.5) |
| Active vitamin D (%) | 40 (65.6) |
| P binders (%) | |
| Ca-acetate | 45 (73.7) |
| Sevelamere-HCl | 8 (13.1) |
| No. of P binders | 5 (8.2) |
| Oral iron (%) | 42 (68.9) |
| Erythropoietin (%) | 34 (55.7) |
| Erythropoietin dosage (u/week) | 8000±2500 |
CCB, calcium channel blockers; RAS, renin angiotensin system.
Laboratory findings of patients.
| Parameters | n=61 |
|---|---|
| Hemoglobin (g/dl) | 11.5±2 |
| Hemoglobin<10g/dl (n, %) | 14 (23) |
| Transferrin saturation (%) | 22±14 |
| Ferritin (ng/ml) | 296±375 |
| Glucose (mg/dl) | 104±22 |
| Serum creatinine (mg/dl) | 9.6±1.1 |
| Serum albumin (g/dl) | 3.8±0.4 |
| C-reactive protein (mg/dl) | 1.5±2.1 |
| Alkaline phosphatase (U/L) | 127±88 |
| Calcium (Ca) (mg/dl) | 9.2±0.7 |
| Phosphorus (P) (mg/dl) | 5.0±1.3 |
| Ca×P product (mg2/dl2) | 47±12 |
| Intact parathormone (pg/ml) | 481±328 |
| Total cholesterol (mg/dl) | 188±45 |
| Triglyceride (mg/dl) | 176±106 |
| LDL-Chol (mg/dl) | 112±35 |
| HDL-Chol (mg/dl) | 40±13 |
| Kt/V | 2.1±0.5 |
| Cre Cl (L/week/1.73m2) | 65.7±21 |
| RRF≥100ml/day (n, %) | 21 (34.4) |
| MIS* | 4.1±1.9 |
| FGF-23 (pg/ml) | 26.3±8.3 |
Laboratory and echocardiographic findings in respect to FGF-23.
| Parameters | FGF-23≤23.5pg/mln=31 | FGF-23>23.5pg/mln=30 | P |
|---|---|---|---|
| Hemoglobin (g/dl) | 12.1±2 | 11.0±2 | 0.017 |
| Transferrin saturation (%) | 24.9±16.8 | 19.5±10.8 | 0.042 |
| Ferritin (ng/ml) | 229±199 | 384±516 | 0.279 |
| Glucose (mg/dl) | 107.3±27.2 | 99.5±14.7 | 0.094 |
| Serum creatinine (mg/dl) | 9.5±1.9 | 9.7±2 | 0.608 |
| Serum albumin (g/dl) | 3.8±0.4 | 3.7±0.5 | 0.371 |
| C-reactive protein (mg/dl) | 1.6±2.4 | 1.5±1.9 | 0.665 |
| Alkaline phosphatase (U/L) | 128±71 | 132±109 | 0.583 |
| Calcium (Ca) (mg/dl) | 9.4±0.6 | 9.0±0.7 | 0.006 |
| Phosphorus (P) (mg/dl) | 4.9±1.2 | 5.2±1.5 | 0.374 |
| Ca×P product (mg2/dl2) | 46±12 | 47±14 | 0.900 |
| Intact parathormone (pg/ml) | 480±328 | 500±349 | 0.863 |
| Total cholesterol (mg/dl) | 194±49 | 182±42 | 0.329 |
| Triglyceride (mg/dl) | 176±104 | 188±110 | 0.846 |
| LDL-C (mg/dl) | 120±37 | 104±32 | 0.072 |
| HDL-C (mg/dl) | 37±11 | 41±13 | 0.199 |
| Kt/V | 2.2±0.5 | 2.0±0.4 | 0.029 |
| CreCl (L/week/1.73m2) | 67.6±22.6 | 65.1±19.3 | 0.697 |
| RRF≥100cc (%)* | 38.7 | 30.0 | 0.655 |
| MIS | 3.9±1.8 | 4.1±2 | 0.395 |
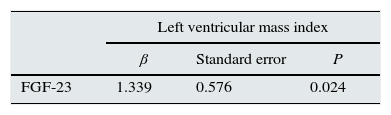
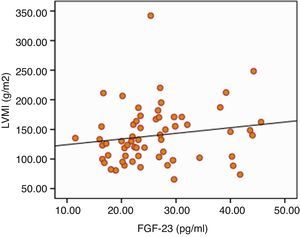
| LVMI (g/m2) | 128±35 | 150±39 | 0.023 |
| LVH (%)* | 58 | 80 | 0.116 |
| EF (%) | 61.0±3.4 | 58.9±9 | 0.661 |
| LVDD (%)* | 76.7 | 80 | 1.0 |
| LVESD (cm) | 3.0±0.3 | 3.2±0.6 | 0.510 |
CreCl, creatinine clearance; EF, ejection fraction; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; LVDD, left ventricular diastolic disfunction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; MIS, malnutrition inflammation score; PDT, posterior wall thickness; RRF, renal residual fraction.
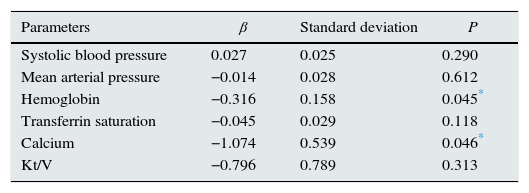
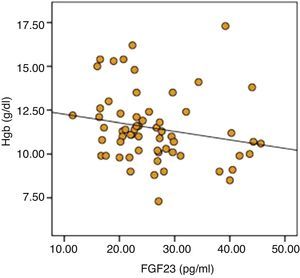
The parameters that were statistically significantly different between two groups were included in by multiple linear regression models to determine the effect of independent variables on FGF-23. As a result of analysis, significant negative relationship of serum calcium (r=0.057, P=0.046) and hemoglobin (r=0.199, P=0.045) was sustained (Table 6). Distribution graphics of statistical relation between FGF-23 and hemoglobin concentration were shown in Fig. 1.
Logistic regression analysis of factors associated with FGF-23.
The results of ECHO parameters were compared in two groups and LVMI was found to be statistically significantly higher in high FGF-23 group (128±35 versus 150±39g/m2, P=0.023) but prevalence of LVH was not statistically different between the groups (58% versus 80%, P=0.116). There were also no differences with regard to other ECHO parameters between two groups. Linear regression analysis was applied to investigate the effect of the serum levels of FGF-23 on LVMI and the relationship was found to be statistically significant (r=0.290, P=0.024) (Table 7). The distribution graph of the relationship between LVMI and FGF-23 can be seen in Fig. 2.
In this study, serum levels of FGF-23 were found to be positively correlated with LVMI. This correlation is likely to be caused by the effects of FGF-23 on left ventricular mass and function, independent from serum calcium, phosphorus, and parathormone levels.
Increased FGF-23 level was shown to be associated with LVMI and LVH in stage II–IV CKD (4.19) and chronic HD patients20,21. Increased FGF-23 level has also been suggested to be a mortality predictor in HD patients3,22, and this situation was thought to be associated with LVMI23. In a study investigating the relationship between FGF-23 levels and LVH in 62 PD patients and 30 healthy controls, serum FGF-23 levels were found to be significantly higher in PD patients compared to control subjects and also in PD patients with LVH compared to PD patients without LVH. FGF-23 levels were also positively correlated with LVMI24. In our study we observed similar association between FGF-23 and LVH in PD patients. There was no difference in other ECHO parameters between patients in high and low FGF-23 groups.
Although observational data supports the possible relationship between the FGF-23 and CVD and CV mortality, insufficient evidence exist about the exact cause of this association. There is not enough data about the physiological effect of FGF-23 in extra-kidney tissue. FGF-23 receptors exist in CV system and other tissues without klotho co-receptor25. This condition, has led to speculation that elevated FGF-23 level observed in progressive renal disease has no direct effect on these tissues but recent data suggests the direct effect of FGF-23 on the CV system26. In a recent experimental study, FGF-23 was shown to cause pathological hypertrophy in rat cardiomyocytes by “calcineurin-nuclear factor of activated T cells” without klotho and treatment with FGF-blockers reduced LVH in experimental models of chronic renal failure27. LVH caused by FGF-23 independent from klotho is explained by two mechanisms. First, longstanding high-FGF-23 level as in CRF, may cause the attachment of FGF to FGF-23 receptor. Although this attachment has lower affinity, exposure of the heart tissue to FGF-23, may be enough for the development of LVH. In CKD klotho expression in the kidney and parathyroid gland is reduced. This may cause the attachment of FGF-23 to FGF-23 receptors in other tissue leading to development of cardio toxicity in CRF. In the second mechanism attachment of FGF-23 to specific cardiac FGF receptors like FGF receptor-4 even in the absence of klotho may contribute to development of LVH22. In a study, besides inducing LVH, elevated FGF-23 has been shown to adversely affect the mechanics of left ventricle in HD patients28. The data of an experimental study showed that FGF23 may have additional effects on the heart, in addition to hypertrophy, specifically related to calcium handling and cardiac contractility29. In a population study, non-sudden cardiac death is found to be associated with high FGF-2330. Recently in the meta-analysis of prospective cohort studies, a relationship between FGF-23 and all-cause mortality and cardiovascular events was observed31. However, additional studies are required to determine FGF-23 receptors that mediate the cardiac effects of FGF-23.
Nevertheless, the presence of cardiac hypertrophy and vascular calcification is interesting in patients with moderate CKD (stages III–IV). These patients do not have hemodynamic and inflammatory stress associated with chronic dialysis treatment and hyperphosphatemia is not obvious but FGF-23 levels are already increased32. This observation suggests that FGF-23 and phosphate might promote distinct mechanisms of cardiovascular toxicity. Indeed, animal models implicate high serum phosphate as a mechanism of vascular calcification and endothelial dysfunction, whereas high levels of FGF-23 are implicated in left ventricular hypertrophy33. FGF-23 may increase CVD by decreasing the serum 1-25(OH)2 vitamin D levels. In this regard the data obtained from HD supports the survival benefit of active vitamin D therapy34. On the other hand the idea that FGF-23 has a direct toxic effect on CV system can only be excluded by prospective trials showing the normalization of FGF-23 by active vitamin D therapy.
Anemia is one of the common complications of CKD and is associated with mortality and cardiovascular events, even after accounting for CKD stage and other cardiovascular risk factors35. In addition, the effect of anemia on CV disease and LVH is well known in CKD patients9. Interestingly in this study significant negative correlation was determined between FGF-23 and hemoglobin concentration and TSAT. Furthermore in high FGF-23 group although statistically insignificant, number of patients having hemoglobin levels lower than 10g/dl and using erythropoietin was higher. Also in regression analysis the negative relation between FGF-23 and hemoglobin is persisted. A study, in HD patients no correlation was detected between hemoglobin and FGF-2336. However, in HD patients especially in patients with ischemic heart disease and heart failure, higher hemoglobin levels have been shown to increase the risk of mortality and morbidity37. For these reasons, the effect of relationship between FGF-23 and anemia on the CV system must be determined in further studies.
A relation between iron and FGF-23 metabolism is mentioned in a few studies. In human and animals with autosomal dominant hypophosphatemic rickets (ADHR) a correlation between elevated FGF-23 with low serum iron was detected. However, in healthy controls, low serum iron was correlated with elevated c-terminal FGF-23 (cFGF-23), but not with iFGF-23. This may suggest that cleavage maintains homeostasis despite increased FGF-23 expression10,38. Similar results were also obtained in patients with X-linked hypophosphatemia39. In studies in children and the elderly population also a relationship between FGF-23 and low serum iron level was detected40,41. In studies, it is emphasized that iron deficiency is an important stimulator of FGF-23 transcription. At the same time, iron is thought to be the cofactor of enzymes taking part in the degradation of intact FGF-23 and thought to have a role in the excretion of degraded FGF-23 parts10,38–41. In another study, the CKD patients with iron deficiency and autosomal dominant rickets showed similar molecular pathophysiology and osteocyte dysfunction is suggested to be increased by iFGF-23 level42. Despite major advances in understanding FGF23 biology, fundamental aspects of FGF23 regulation in healthy subjects and in CKD remain mostly unknown.
In many human and animal studies effect of different iron preparations on FGF-23 and phosphate levels is investigated. Iron polymaltose infusions were demonstrated to raise i-FGF23 concentrations causing hypophosphatemia and osteomalacia in individuals with normal renal function43. In a study intravenous (IV) ferric carboxymaltose (FCM) and iron dextran (ID) were used. C-terminal FGF-23 was decreased after the use of both drugs. But only in FCM group iFGF-23 increased temporarily and serum phosphorous is decreased44. Besides this in HD patients PTH suppression and rise in iFGF-23 level after IV low molecular weight ID treatment was observed45. In another study in HD patients iFGF-23 level was found to be negatively associated to intravenous iron sucrose use independently from serum ferritin and iron levels. But in this study there was no data about the association of hemoglobin and iFGF-2346. Researchers think some carbohydrate components in iron preparations to inhibit FGF-23 degradation in osteocytes to cause a transient raise in iFGF-2344–46. In another study iron isomaltose and FCM given intraperitoneally were shown to have no effect on plasma iFGF23 levels both in normal and uremic rats47. In a recent double blinded, placebo-controlled, randomized trial, rise in hemoglobin levels and decline in serum phosphate and iFGF-23 levels were detected after the use of oral ferric citrate for 12 weeks48. Also in an experimental study, FGF-23 is expressed to have negative effect on erythrocyte production and differentiation49. In our study group, none of the patients were under intravenous iron treatment. Lower than the half of the patients was using oral iron drugs and the percentage was similar in both groups. Negative correlation was detected between iFGF-23 and hemoglobin levels independent from serum ferritin, TSAT and oral iron usage in patients. The results of our studies and other studies suggest an association between disease and FGF-23 metabolism.
Clinical study number in which FGF-23 concentrations are measured is increasing. But studies in which iFGF-23 (kainos; immutopics) and cFGF-23 (immutopics) measurements are compared are inadequate. In a study intra-assay variation was found to be good in all three assays but in terms of inter-assay and linearity, kainos iFGF-23 and cFGF-23 assays were found to be better50. In another study strong positive correlation was determined between iFGF assays and cFGF assay in the HD patients51. In a study an important diurnal variation is detected in iFGF-23 so the use of cFGF-23 is thought to be more useful in clinical practice52. Also both immunotrophic assays are thought to be affected from ferritin levels but this situation does not occur in kainos iFGF-23 assay53. As a result of these data we think the use of both iFGF-23 assay and cFGF-23 assay together may be suitable.
There are some limitations in this cross sectional study. Due to the design of the study left ventricular function and geometry was assessed by echocardiography and changes that have occurred over time are shown. But iFGF-23 levels were measured once and possibility of change in serum iFGF-23 level during the time period could not be known. However, only iFGF-23 levels were measured. Otherwise, this is a single-centered study, the number of patients and race diversity is limited. Due to the cross-sectional design of the study we can only talk about a relationship based on the data but not a causal relationship so interpretations should be made carefully.
In conclusion, data presented in this study and recent publications about the relationship between CV risk factors and anemia with FGF-23, suggests a wider role of this marker in normal biological systems. Well-designed prospective randomized trials are needed to investigate its exact role in the pathogenesis of CVD and anemia in CKD.
Conflicts of interestThe authors have no conflicts of interest to declare.