Type 2 diabetes mellitus and chronic kidney disease (CKD) are conditions which have a high prevalence in individuals ≥65 years of age and represent a major public health problem.
ObjectivesTo determine the prevalence of CKD, its categories and its relationship with various demographic and clinical factors in elderly patients with type 2 diabetes mellitus in Spain.
MethodsObservational, cross-sectional, multicenter, Spanish epidemiological study. Patients with known type 2 diabetes mellitus, age ≥65 years of age treated in Primary Care were included. We collected demographic, anthropometric and analytical variables from the previous 12 months, including the albumin-to-creatinine ratio and estimated glomerular filtration rate to evaluate renal function.
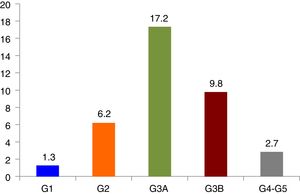
ResultsThe prevalence of CKD was 37.2% (95% CI, 34.1–40.3%), renal failure was 29.7% (95% CI, 26.8–32.6%) and increased albuminuria was 20.6% (95% CI, 17.3–23.9%), moderately increased albuminuria was 17.8% (95% CI, 14.7–20.9%) and severely increased albuminuria was 2.8% (95% CI, 1.4–4.2%). In turn, the prevalence of CKD categories were: G1 1.3% (95% CI, 0.6–2%), G2 6.2% (95% CI, 4.6–7.8%), G3a 17.2% (95% CI, 14.8–19.6%), G3b 9.8% (95% CI, 7.9–11.7%), G4 2% (95% CI, 1.1–2.9%) and G5 0.7% (95% CI, 0.2–1.2%).
In the multivariate analysis, after adjusting for the remaining variables, CKD was associated with elderly age (OR 5.13, 95% CI, 3.15–8.35), high comorbidity (OR 3.36, 95% CI, 2.2–5.12) and presence of antihypertensive treatment (OR 2.43, 95% CI, 1.48–4.02).
ConclusionsCKD is frequent in the diabetic population ≥65 years of age and is associated with elderly age, high comorbidity and with treated hypertension. No relationship has been found with gender and time in years since onset of diabetes.
La diabetes mellitus tipo 2 y la enfermedad renal crónica (ERC) son afecciones de elevada prevalencia en personas ≥ 65 años y constituyen un importante problema de salud pública.
ObjetivosConocer la prevalencia de la ERC, sus categorías y su relación con diversos factores demográficos y clínicos, en pacientes ancianos con diabetes mellitus tipo 2 en España.
MétodosEstudio epidemiológico, observacional, transversal, multicéntrico, ámbito nacional. Se incluyeron pacientes con diabetes mellitus tipo 2 conocida, edad ≥ 65 años atendidos en Atención Primaria. Se recogieron variables demográficas, antropométricas y analíticas de los últimos 12 meses, incluyendo el cociente albúmina-creatinina y el filtrado glomerular estimado para evaluar la función renal.
ResultadosLa prevalencia de ERC fue del 37,2% (IC95%, 34,1-40,3%), de insuficiencia renal del 29,7% (IC95%, 26,8-32,6%) y de elevación de la albuminuria del 20,6% (IC95%, 17,3-23,9%), moderadamente elevada 17,8% (IC95%, 14,7-20,9%), severamente elevada 2,8% (IC95%, 1,4-4,2%). La prevalencia de las categorías de ERC fueron: G1 1,3% (IC95%, 0,6-2%), G2 6,2% (IC95%, 4,6-7,8%), G3a 17,2% (IC95%, 14,8-19,6%), G3b 9,8% (IC95%, 7,9-11,7%), G4 2% (IC95%, 1,1-2,9%) y G5 0,7% (IC95%, 0,2-1,2%).
En el análisis multivariante, después de ajustar por el resto de variables, la ERC se asoció a mayor edad OR 5,13, (IC95%, 3,15-8,35), alta comorbilidad OR 3,36 (IC95%, 2,2-5,12) y la presencia de tratamiento antihipertensivo OR 2,43 (IC95%, 1,48-4,02).
ConclusionesLa ERC es frecuente en la población diabética ≥ 65 años y se asocia con mayor edad, alta comorbilidad e hipertensión tratada. No se ha encontrado asociación con el género y años de evolución de la diabetes.
Chronic kidney disease (CKD) is a common comorbidity in patients with type 2 diabetes mellitus (DM2) and in both conditions the prevalence is increasing.
Worldwide epidemiological data show that DM2 is one of the main epidemics of the 21st century. Approximately 415 million people (between 20 and 79 years old) suffered from this condition in 2015, of which 94.2 million (22.7% of the total) were between 65 and 79 years old. In addition, 318 million people were at a high risk of developing DM2. It is estimated to that DM2 will affect 642 millions in the 2040s (one out of ten adults will have the disease) and almost a third of them will be between 65 and 79 years. Sources from the International Diabetes Federation1 indicates that DM2 is more common in men than in women.
According to the Di@bet.es study,2 the prevalence of DM2 in Spain increases with age and is higher in men than in women except in those older than 75 years, with a prevalence of 40% (41.3% in women and 37.4% in men) while in the 61–75 years group, between, the prevalence is 42.4% in men and 29.8% in women. The data on DM in our country in people over 60 years, which is between 18 and 25%, are similar to the United States population of 65 or older, which was 25.9% in 2012, affecting 11.2 million people.3
Chronic kidney disease (CKD) is associated with an increase in cardiovascular morbidity and mortality, mortality from any cause and progression of kidney disease, both in the general population and in patients with DM2.4 The prevalence of CKD in adult populations in Western countries varies from 5.8% in Poland to 14.8% in the United States.5,6 The prevalence of CKD increases with age and in people with diabetes, in which the prevalence of CKD varies between 34.7 and 45.4% according to populations.6–10 In our country, according to data from the Epirce study,11 CKD affects 9.16% of the adult population over 20 years of age (23.7% in people over 65 years of age) and the prevalence of CKD in people with diabetes reaches 27.9% according to the Percedime2 study,12 with an average age of 66.8 years.
The present work is part of the ESCADIANE study (Study of the characteristics of elderly patients with diabetes in Spain) designed by the Group of Primary Care of the Spanish Society of Diabetes. The main objective is to evaluate the prevalence of CKD and its different stages in patients with diabetes aged ≥65 years in Spain and determine their relationship with various demographic factors (gender, age) and clinical features: glycemic control, years of evolution of their DM, anthropometric measures, current treatments, hypoglycemic episodes, degrees of dependence and comorbidity.
MethodsStudy designThe present work is part of the ESCADIANE study, an epidemiological, observational, cross-sectional, multicenter, national study, that aims to know the health status of diabetics patients older that 65 years in Spain.
The study population includes patients being followed by physicians researchers, with the diagnosis of DM2 (following the criteria of the American Diabetes Association 2011)13 being recorded in the clinical history, with an age ≥65 years and with the necessary data to complete the questionnaire. The participants in the study had to sign the informed consent (the participant or his legal representative) and they were not included in any other type of epidemiological study. Patients in a terminal situation and those who did not meet the inclusion criteria were excluded, according to the evaluation committee. The study was qualified as an observational-type EPA-OD study by the Spanish Agency of Medications and it was evaluated and approved by the CEIC of Aragón on January 30, 2013.
ProcedureHealth professionals of Primary Health Care from the different autonomous regions participated as researchers. The distribution of participants was proportional to the population of each of the regions of Spain.
Each medical researcher was asked to select twelve of his patients with DM of 65 years or older. A sequence of random numbers was provided through which the 12 patients in a list should be selected.
The data collection was developed between October 2014 and April 2015, in the context of usual clinical practice.
Finally, 82 primary care physicians of the ESCADIANE study, throughout the Spanish geography, included 939 patients that were used for the analysis of kidney disease.
MeasurementsThe following demographic and anthropometric variables were collected: age, gender, weight (kg), height (cm), body mass index (BMI) (kg/m2), abdominal perimeter (cm) and waist circumference/height ratio (R W/H).
Also recorded was, the presence of cardiovascular risk factors (arterial hypertension [HTN] and dyslipidemia), cardiovascular diseases and other events with a confirmed diagnosis and dated in the clinical history: cerebrovascular disease and sequelae (hemiplegia or paraplegia), coronary heart disease (myocardial infarction, angina pectoris, coronary interventions), heart failure, symptomatic peripheral arteriopathy, retinopathy, neuropathy, chronic respiratory disease, rheumatic diseases, mild or severe liver disease, peptic ulcer, neoplasms (lymphomas, leukemias, solid tumors and presence of metastasis), advanced CKD or dialysis and chronic cognitive impairment.
Clinical and analytical variables were also collected: years of evolution of DM2, hypoglycemia recorded in the clinical history requiring medical assistance, blood pressure (mmHg), smoking habits (smoker, ex-smoker, non-smoker), alcohol consumption (drinker, ex-drinker, no drinker), medications taken by the patient at the time of data collection (antidiabetic, antihypertensive, lipid-lowering, antiplatelet agents and anticoagulants), Barthel index, basal glycemia (mg/dl), glycosylated hemoglobin (HbA1c,%), lipid profile (mg/dl), serum creatinine (mg/dl) and the albumin/creatinine ratio (ACR) (mg/g) in morning urine sample. Blood biochemistry was performed in the reference laboratory of each researcher.
Obesity was defined as BMI≥30kg/m2. Abdominal obesity, as waist circumference >102cm (men) or >88cm (women). Hypertension was defined as blood pressure ≥140/90mmHg or the use of antihypertensive medications. Hyperlipidemia was considered if total cholesterol was >200mg/dl or LDL-C cholesterol >100mg/dl or HDL cholesterol <40mg/dl in men and <50mmol/kg in women or triglycerides >150mg/dL or lipid-lowering drug therapy, according to the American Diabetes Association 2011.13
The level of dependence was evaluated by the Barthel Index14; patients were classified according to the score in: independent (100 points), mild dependence (91–99 points), moderate dependence (61–90 points), severe or serious dependence (21–60 points) and total dependence (0–20 points). Comorbidity was assessed using original Charlson Index.15 Patients were classified according to the score of comorbidity in absence: 0–1 points, low: 2 points and high: ≥3 points.
The kidney function was defined according to the criteria of the Kidney Disease: Improving Global Outcomes KDIGO 201216 and the Spanish Consensus Document for the detection and management of CKD17; the presence of CKD was considered in patients with an estimated renal glomerular filtration rate (eGFR) <60mL/min/1.73m2 and/or the presence of kidney damage (defined as a Albumin/Cr (ACR) in urine greater than or equal to 30mg/g); the glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease-Epidemiology Collaboration equation.18 Renal insufficiency (RI) was considered if the eGFR <60mL/m/1.73m2. Albuminuria was defined as a ACR of 30mg/g or more, regardless of gender. The categories of albuminuria were: normal A1 or mild increase (ACR<30mg/g), A2 moderate increase (ACR 30–299mg/g) and A3 severe increase (ACR≥300mg/g). The categories of CKD were defined as follows: G1 (eGFR≥90ilamL/min/1.73m2 and ACR≥30mg/g); G2 (eGFR 60–89mL/min/1.73m2 and ACR≥30mg/g); G3a (FGe 45–59mL/min/1.73m2 regardless of ACR); G3b (FGe 30–44mL/min/1.73m2 regardless of ACR); G4 (FGe 15–29mL/min/1.73m2 independently of the ACR) and G5 (FGe<L15mL/min/1.73m2 independently of the ACR). The eGFR categories (G1 to G5) are defined with the same eGFR intervals independently of the ACR. The final analysis of the study was performed using a single determination of creatinine and albuminuria, as no further values were available. The variable race was not taken into account, given the characteristics of the population of our environment, where the Caucasian race (99%) is clearly predominant, especially in this age group.
Statistic analysisA descriptive analysis of the demographic, anthropometric, analytical and comorbidities variables was performed. Values of continuous variables have been described by central, non-central tendency and dispersion. Qualitative variables by absolute and relative frequency distributions with 95% CI. For comparison of quantitative variables and qualitative variables, it was proved, by the Kolmogorov–Smirnov test, whether quantitative variables followed a normal distribution in the categories of qualitative variables. Parametric tests (t-Student test or ANOVA) were used in the case of normality, otherwise nonparametric tests (Mann Whitney U or Kruskal Wallis test) were used, all of them as independent samples.
The Chi-square test was used to compare proportions and the Yates correction was used as necessary. In case of multiple comparisons, p-values were corrected by the Bonferroni method. The degree of association between the demographic and clinical characteristics of the patients and the presence or absence of CKD was established by multivariate logistic regression. Statistical significance was established at 0.05 when hypotheses were tested. The analysis and processing of the data was done using the IBM SPSS Statistics Software v.23.
ResultsThe study included 939 patients from a total of 82 primary care physicians distributed throughout the Spanish geography; 929 provided the creatinine value and were included in the analysis of kidney disease. Of these, 608 (65.5%) had a determination of the ACR. The average age of the participants was 76.4 years (standard deviation [SD] 6.7), 52.9% (95% CI: 49.7–56.1) were women. The demographic, anthropometric and clinical characteristics of the participants are shown in Table 1.
Characteristics of patients with DM2 over 65 years (n=939).
| Total | Men | Women | p-Value* | |
|---|---|---|---|---|
| N (%) | 939 | 442 (47.1%) | 497 (52.9%) | |
| Mean (SD) | ||||
| Age (years) | 76.4 (6.7) | 75.8 (6.3) | 77 (7) | 0.006 |
| Years evolution | 11.6 (7.9) | 11.4 (7.3) | 11.9 (8.4) | 0.337 (ns) |
| BMI (kg/m2) | 29.5 (4.6) | 29.1 (4) | 29.8 (5.1) | 0.019 |
| Waist circumference (cm) | 101.2 (12.2) | 104 (11) | 98.7 (12.6) | 0.000 |
| RW/H | 0.63 (0.08) | 0.62 (0.07) | 0.64 (0.09) | 0.002 |
| Barthel Index | 92.43 (17.6) | 94.9 (15.5) | 90.2 (18.9) | 0.000 |
| Charlson Index | 2.7 (1.9) | 2.9 (2) | 2.5 (1.8) | 0.001 |
| Glycemia (mg/dl) | 137 (39.6) | 138.7 (38.8) | 135.6 (40.3) | 0.236 (ns) |
| HbA1c (%) | 7.04 (1.16) | 7.03 (1.13) | 7.05 (1.19) | 0.732 (ns) |
| SBP (mmHg) | 135.9 (15.4) | 136.1 (14.8) | 135.7 (15.9) | 0.731 (ns) |
| DBP (mmHg) | 74.7 (9.9) | 75.2 (9.7) | 74.3 (10.1) | 0.205 (ns) |
| Total cholesterol (mg/dl) | 173.8 (37.2) | 164.3 (34.6) | 182.1 (37.5) | 0.000 |
| HDL-cholesterol (mg/dl) | 50.9 (14.8) | 47.1 (13.9) | 54.2 (14.8) | 0.000 |
| LDL-cholesterol (mg/dl) | 95.6 (32.2) | 91 (30.7) | 99.7 (33.1) | 0.000 |
| Triglycerides (mg/dl) | 139 (70.3) | 132.6 (71.3) | 144.8 (68.9) | 0.008 |
| Creatinine (mg/dl) | 0.97 (0.47) | 1.09 (0.51) | 0.87 (0.40) | 0.000 |
| eGFR (mL/m/1.73m2) | 70.4 (19.4) | 71.3 (19.0) | 69.6 (19.7) | 0.187 (ns) |
| ACR (N=608) (mg/g) | 39.9 (103.8) | 48.1 (113.3) | 32.2 (93.5) | 0.060 (ns) |
| Smoking habits (%) | ||||
| Smoker | 62 (6.7%) | 49 (79%) | 13 (21%) | 0.001 |
| Former smoker | 214 (23%) | 195 (91.1%) | 19 (8.9%) | |
| Non smoker | 656 (70.3%) | 195 (29.7%) | 461 (70.3%) | |
| Use of alcohol (%) | ||||
| Drinker | 212 (22.7%) | 178 (84%) | 34 (16%) | 0.001 |
| Ex-drinker | 67 (7.1%) | 63 (94%) | 4 (6%) | |
| Non-drinker | 654 (70.2%) | 199 (30.4%) | 455 (69.6%) | |
ACR: albumin creatinine ratio in urine; SD: standard deviation; HbA1c: glycosylated hemoglobin; BMI: body mass index; ns: not significant; SBP, DBP: systolic, diastolic blood pressure; R W/H: waist height ratio.
Compared with men, women had a higher mean age, BMI, waist circumference/height ratio, worse lipid profile, and higher degree of dependency. By contrast, men had higher creatinine levels, more comorbidity and worse habits in terms of tobacco and alcohol. No differences were found in terms of years of evolution of DM2, glycemia, HbA1c, blood pressure, glomerular filtration rate and ACR in urine.
Prevalence and characteristics of chronic kidney diseaseThe prevalence of CKD was 37.2% (95% CI, 34.1–40.3%), that of RI was 29.7% (95% CI, 26.8–32.6%) and high albuminuria was present in 20.6% (95% CI, 17.3–23.9%): moderately elevated 17.8% (95% CI, 14.7–20.9%) and severely elevated 2. 8% (95% CI, 1.4–4.2%). The prevalence of CKD according to categories following the KDIGO guidelines and the Spanish consensus document for the detection and management of CKD17 is shown in Fig. 1. Table 2 describes the characteristics of patients according to the presence of CKD.
Prevalence of CKD, classified into categories following the KDIGO guidelines. CKD categories. G1: eGFR≥90mL/m/1.73m2 and ACR≥30mg/g; G2: eGFR 60–89mL/m/1.73m2 and ACR≥30mg/g; G3A: eGFR 45–59mL/m/1.73m2; G3B: eGFR 30–44mL/m/1.73m2; G4 eGFR 15–29mL/m/1.73m2; G5 eGFR<15mL/m/1.73m2; eGFR: estimated glomerular filtration; ACR: albumin/creatinine ratio.
Characteristics of patients with and without kidney disease (n=929).
| Total | Kidney disease | No kidney disease | p-Value* | |
|---|---|---|---|---|
| N (%) | 929 | 346 (37.2%) | 583 (62.8%) | |
| Years of evolution (mean, SD) | 11.6 (7.9) | 12.8 (8.5) | 10.9 (7.4) | <0.001 U Mann–Whitney |
| Gender, N (%) | ||||
| Males | 437 (47) | 159 (36.4) | 278 (63.6) | ns |
| Females | 492 (53) | 187 (38) | 305 (62) | |
| Age groups N (%) | ||||
| 65–74 a | 390 (42.0) | 95 (24.4) a | 295 (75.6) a | <0.001 |
| 75–84 a | 404 (43.5) | 168 (41.6) b | 236 (58.4) b | |
| ≥85 | 135 (14.5) | 83 (61.5) c | 52 (38.5) c | |
| BMI (kg/m2), N (%) | ||||
| ≤30 | 560 (60.3) | 202 (36.1) | 358 (63.9) | ns |
| >30 | 368 (39.7) | 144 (39.1) | 224 (60.9) | |
| Central obesity, n (%) | ||||
| Yes | 626 (68.3) | 238 (38) | 388 (62) | ns |
| No | 290 (31.7) | 101 (34.8) | 189 (65.2) | |
| R W/H, N (%) | ||||
| ≤0.55 | 122 (13.3) | 40 (32.8) | 82 (67.2) | ns |
| >0.55 | 794 (86.7) | 299 (37.7) | 495 (62.3) | |
| HbA1c, N (%) | ||||
| <7% | 534 (57.7) | 188 (35.2) | 346 (64.8) | ns |
| 7–8.5% | 289 (31.2) | 108 (37.4) | 181 (62.6) | |
| >8.5% | 103 (11.1) | 48 (46.6) | 55 (53.4) | |
| Macrovascular disease, N (%) | ||||
| Yes | 266 (28.8) | 123 (46.2) | 143 (53.8) | <0.001 |
| No | 658 (71.2) | 222 (33.7) | 436 (66.3) | |
| Microvascular disease, N (%) | ||||
| Yes | 160 (17.3) | 70 (43.8) | 90 (56.2) | ns |
| No | 763 (82.7) | 275 (36) | 488 (64) | |
| Hypoglycemia, N (%) | ||||
| Yes | 116 (12.5) | 57 (49.1) | 59 (50.9) | <0.005 |
| No | 812 (87.5) | 289 (35.6) | 523 (64.4) | |
| Insulin | ||||
| Yes | 234 (25.2) | 112 (47.9) | 122 (52.1) | <0.001 |
| No | 693 (74.8) | 234 (33.8) | 459 (66.2) | |
| OAD | ||||
| Yes | 763 (82.3) | 268 (35.1) | 495 (64.9) | <0.003 |
| No | 164 (17.7) | 78 (47.6) | 86 (52.4) | |
OAD: oral antidiabetics; SD: standard deviation; KD: kidney disease; HbA1c: glycosylated hemoglobin; BMI: body mass index; ns: not significant; R W/H: waist height ratio.
In variables with more than two categories, the letters a, b or c that differ indicate in which categories the differences are statistically significant. Comparison by rows.
Bivariate statistics comparing patients with CKD and without CKD, no statistically significant differences were found in gender, peripheral obesity, abdominal obesity, waist circumference/height, glycemic control and the presence of microvascular diseases. However, patients with CKD had a longer duration of diabetes, more age (higher proportion of CKD in older age groups), presence of macrovascular disease, more hypoglycaemia, greater insulin utilization and less use of oral antidiabetic drugs, than those without CKD. The prevalence of kidney disease increased according to the degree of dependence (35.8% independence-mild dependence vs. 56.3% moderate-severe dependence p<0.0001) and also according to degree of comorbidity, 24.7%. 54.2%, p<0.001, according to the absence of morbidity or high morbidity respectively.
The prevalence of CKD considering only the patients that provide albuminuria determination was 39.5% (95% CI 35.6–43.4%).
The prevalences of the different categories of eGFR (G1, G2, G3A, G3B, G4–G5) were: 13.9% (95% CI, 11.7–16.1%), 56.4% (95% CI, 53.2–59.6%), 17.2% (95% CI, 14.8–19.6%), 9.8% (95% CI, 7.9–11.7%) and 2.7% (95% CI, 1.7–3.7%) respectively.
The deterioration of renal function was associated with a longer duration of diabetes, older age, worse glycemic control, presence of micro- or macrovascular disease, greater number of hypoglycaemia episodes, greater degree of dependence and greater presence of comorbidity. No statistically significant differences were found regarding CKD, between the different anthropometric measurements and gender.
Regarding treatment, 25.2% of patients were on insulin, the use of insulin was significantly increased in patients with deterioration of renal function. Regarding oral antidiabetics (OAD), 70.2% used metformin, 28.7% inhibitors of the enzyme dipeptidyl peptidase-4 (IDPP-4) and 16.3% sulfonylureas (SU) (glibenclamide 1.2%, 36.4% of them in categories 3–5 of ER) alone or in combination. The use of metformin and SU decreases as renal function worsens; in patients with eGFR <60mL/m/1.73m2, 24.3% use metformin and 26.4% sulfonylureas and in those with eGFR<30mL/m/1.73 m2 only 0.8% (95% CI, 0.1–1.5%) and 2.6% (95% CI 0.1–5.1%) use metformin and SU respectively. Likewise, 1.7% (95% CI, 0.3–3.1%) of the patients used thiazide diuretics for the treatment of their HTN with eGFR<30mL/m/1.73m2. In these situations, the use of drugs (metformin, sulfonylureas) would be formally contraindicated for safety reasons or not recommended due to lack of efficacy as in the case of thiazides. Almost 70% of patients include an inhibitor of the renin–angiotensin system in their treatment regimen for hypertension and 65% use statins (Table 3).
Characteristics of patients according to categories of chronic kidney disease (n=929).
| mL/m/1.73m2 | G1 KD eGFR≥90 | G1 no KD eGFR≥90 | G2 KD eGFR 60–89 | G2 no KD eGFR 60–89 | G3A eGFR 45–59 | G3B eGFR 30–44 | G4-5 eGFR <30 | p-Value* |
|---|---|---|---|---|---|---|---|---|
| N=929, KD=346 (37.2%) | 12 (1.3) | 117 (12.6) | 58 (6.2) | 466 (50.2) | 160 (17.2) | 91 (9.8) | 25 (2.7) | |
| Years evolution, M(SD) | 11.7 (8.1) | 11.3 (8.4) | 10.9 (8.3) | 10.9 (7.1) | 12.8 (7.6) | 13.9 (9.9) | 13.6 (9.0) | 0.008 |
| HbA1c (%), M(SD) | 7.5 (1.4) | 7.2 (1.2) | 7 (1.4) | 6.9 (1.0) | 7.07 (1.2) | 7.19 (1.3) | 7.40 (1.2) | ns |
| Age group, n (%) | ||||||||
| 65–74 years | 8 (2.1) | 96 (24.6) a | 20 (5.1) | 199 (51) | 43 (11) a | 17 (4.4) a | 7 (1.8) | <0.001 |
| 75–84 years | 4 (1) | 19 (4.7) b | 33 (8.2) | 217 (53.7) a | 75 (18.6) | 44 (10.9) | 12 (3) | |
| ≥85 years | 0 (0) | 2 (1.5) b | 5 (3.7) | 50 (37) b | 42 (31.1) b | 30 (22.2) b | 6 (4.4) | |
| Gender, n (%) | ||||||||
| Male | 5 (1.1) | 50 (11.4) | 35 (8) | 228 (52.2) | 73 (16.7) | 32 (7.3) | 14 (3.2) | ns |
| Female | 7 (1.4) | 67 (13.6) | 23 (4.7) | 238 (48.4) | 87 (17.7) | 59 (12) | 11 (2.2) | |
| R W/H, n (%) | ||||||||
| ≤0.55 | 1 (0.8) | 20 (16.4) | 6 (4.9) | 62 (50.8) | 18 (14.8) | 8 (6.6) | 7 (5.7) | ns |
| >0.55 | 11 (1.4) | 96 (12.1) | 51 (6.4) | 399 (50.3) | 138 (17.4) | 81 (10.2) | 18 (2.3) | |
| BMI (kg/m2), n (%) | ||||||||
| BMI≤30 | 6 (1.1) | 71 (12.7) | 33 (5.9) | 287 (51.2) | 97 (17.3) | 51 (9.1) | 15 (2.7) | ns |
| BMI>30 | 6 (1.6) | 46 (12.5) | 25 (6.8) | 178 (48.4) | 63 (17.1) | 40 (10.9) | 10 (2.7) | |
| Central obesity, n (%) | ||||||||
| Yes | 9 (1.4) | 81 (12.9) | 42 (6.7) | 307 (49) | 104 (16.6) | 67 (10.7) | 16 (2.6) | ns |
| No | 3 (1) | 35 (12.1) | 15 (5.2) | 154 (53.1) | 52 (17.9) | 22 (7.6) | 9 (3.1) | |
| HbA1c groups, n (%) | ||||||||
| <7% | 7 (1.3) | 63 (11.8) | 37 (18.1) | 283 (53) | 91 (17) | 45 (8.4) | 8 (1.5) a | 0.023 |
| 7–8.5% | 1 (0.3) a | 37 (12.8) | 14 (4.8) | 144 (49.8) | 50 (17.3) | 30 (10.4) | 13 (4.5) b | |
| >8.5% | 4 (1.3) b | 17 (16.5) | 7 (6.8) | 38 (36.9) | 19 (17.8) | 15 (14.6) | 3 (2.9) | |
| Hipoglycemia, n (%) | ||||||||
| Yes | 0 (0) | 12 (10.3) | 7 (6.0) | 47 (40.5) a | 24 (20.7)) | 18 (15.5) a | 8 (6.9) a | 0.005 |
| No | 12 (1.5) | 105 (12.9) | 51 (6.3) | 418 (51.5) b | 136 (16.7) | 73 (9.0) b | 17 (2.1) b | |
| Macrovascular disease, n (%) | ||||||||
| Yes | 3 (1.1) | 27 (10.2) | 20 (7.5) | 116 (43.6) a | 52 (19.5) | 34 (12.8) | 14 (5.3) a | 0.004 |
| No | 9 (1.4) | 90 (13.7) | 38 (5.8) | 346 (52.6) b | 107 (16.3) | 57 (8.7) | 11 (1.7) b | |
| Microvascular disease, n (%) | ||||||||
| Yes | 5 (3.1) a | 26 (16.3) | 12 (7.5) | 64 (40) a | 25 (15.6) | 19 (11.9) | 9 (5.6) a | 0.005 |
| No | 7 (0.9) b | 91 (11.9) | 46 (6) | 397 (52) b | 134 (17.6) | 72 (9.4) | 16 (2.1) b | |
| Treatment with metformin, n (%) | ||||||||
| Yes | 11 (1.7) | 91 (14) | 44 (6.8) | 347 (53.3) a | 110 (16.9) | 43 (6.6) a | 5 (0.8) a | <0.001 |
| No | 1 (0.4) | 26 (9.4) | 14 (5.1) | 117 (42.4) b | 50 (18.1) | 48 (17.4) b | 20 (7.2) b | |
| Sulfonylureas treatment, n (%) | ||||||||
| Yes | 3 (2) | 22 (14.6) | 4 (2.6) | 82 (54.3) | 24 (15.9) | 12 (7.9) | 4 (2.6) | ns |
| No | 9 (1.2) | 95 (12.2) | 54 (7) | 382 (49.2) | 136 (17.5) | 79 (10.2) | 21 (2.7) | |
| Insulin treatment, n(%) | ||||||||
| Yes | 4 (1.7) | 27 (11.5) | 16 (6.8) | 95 (40.6) a | 42 (17.9) | 36 (15.4) a | 14 (6) a | <0.001 |
| No | 8 (1.2) | 90 (13) | 42 (6.1) | 369 (53.2) b | 118 (17) | 55 (7.9) b | 11 (1.6) b | |
| Thiazide treatment for HTN, n (%) | ||||||||
| Yes | 3 (0.8) | 41 (11.4) | 19 (5.3) | 182 (50.7) | 75 (20.9) | 33 (9.2) | 6 (1.7) | ns |
| No | 9 (1.6) | 76 (13.4) | 39 (6.9) | 282 (49.6) | 85 (15) | 58 (10.2) | 19 (3.3) | |
| ACEI/ARA I I treatment, n (%) | ||||||||
| Yes | 5 (0.8) a | 72 (11.4) | 45 (7.1) | 297 (46.9) a | 128 (20.2) | 67 (10.6) | 19 (3) | <0.002 |
| No | 7 (2.4) b | 45 (15.3) | 13 (4.4) | 167 (56.8) b | 32 (10.9) | 24 (8.2) | 6 (2) | |
| Statins treatment, n (%) | ||||||||
| Yes | 5 (0.8) | 78 (12.9) | 39 (6.5) | 297 (49.3) | 108 (17.9) | 59 (9.8) | 17 (2.8) | ns |
| No | 7 (2.2) | 39 (12) | 19 (5.9) | 167 (51.5) | 52 (16) | 32 (9.9) | 8 (2.5) | |
| Albuminuria (mg/g), n (%) N=608 | ||||||||
| <30 | 0 (0) a | 74 (15.4) | 0 (0) a | 294 (61.1) a | 73 (15.2) | 32 (6.7) a | 8 (1.7) | <0.001 |
| 30–299 | 12 (11.1) b | 0 (0) | 52 (48.1) b | 0 (0) b | 23 (21.3) | 16 (14.8) b | 5 (4.6) | |
| ≥300 | 0 (0) b | 0 (0) | 6 (35.3) b | 0 (0) b | 3 (17.6) | 7 (41.2) a | 1 (5.9) | |
| Dependency (Barthel), n (%) | ||||||||
| Severe | 0 (0) | 3 (13) | 3 (13) | 9 (39.2) | 3 (13) | 4 (17.5) | 1 (4.3) | 0.028 |
| Moderated | 1 (2.4) | 2 (4.9) | 1 (2.4) | 14 (34.1) a | 16 (39) a | 6 (14.6) | 1 (2.4) | |
| Mild | 11 (1.3) | 112 (12.9) | 54 (6.2) | 443 (51.2) b | 141 (16.3) b | 81 (9.4) | 23 (2.7) | |
| Comorbidity (Charlson), n (%) | ||||||||
| Absence | 4 (1.4) | 51 (17.3) a | 17 (5.8) | 171 (58) a | 37 (12.5) a | 15 (5.1) a | 0 (0) a | <0.001 |
| Low | 4 (1.7) | 33 (14.2) | 13 (5.6) | 141 (60.8) b | 35 (15.1) | 5 (2.2) b | 1 (0.4) b | |
| High | 4 (1) | 33 (8.4) b | 28 (7.1) | 148 (37.5) b | 87 (22) b | 71 (18) b | 24 (6.1) b | |
SD: standard deviation; KD: kidney disease; eGFR: estimated glomerular filtration; HbA1c: glycosylated hemoglobin; HTN: hypertension; BMI: body mass index; M: mean; ns: not significant; R W/H: waist height ratio.
The categories G4–G5 have been grouped. In variables with more than two categories, (multiple comparisons) the letters a, b, or c indicate in which categories the differences are statistically significant. Comparison of proportions by rows.
Finally, in the multivariate analysis, renal disease was associated with older age OR: 5.13 (95% CI, 3.15–8.35 p<0.001), high comorbidity OR: 3.36 (95% CI, 2.20–5.12 p<0.001) and antihypertensive treatment OR: 2.43 (CI 95%, 1.48–4.02, p<0.001) (Table 4).
Factors associated with CKD in patients with DM2 ≥65 years (n=929).
| Univariate logistic regression model. Without adjusting for the rest of the variables | Multivariate logistic regression model. Adjusted for the rest of the variables | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| DM2 years | 1.03 (1.01–1.05) | 0.001 | 1.00 (0.98–1.03) | 0.667 |
| Age group (65–74 years old) | 0.000 | 0.000 | ||
| 75–84 years | 2.21 (1.63–3) | 0.000 | 2.04 (1.46–2.84) | 0.000 |
| ≥85 years | 4.96 (3.27–7.52) | 0.000 | 5.13 (3.15–8.35) | 0.000 |
| Group HBA1c (<7%) | 0.093 | 0.084 | ||
| 7–8.5% | 1.1 (0.82–1.48) | 0.537 | 0.75 (0.53–1.08) | 0.122 |
| ≥8.5% | 1.61 (1.05–2.46) | 0.029 | 1.31 (0.77–2.24) | 0.318 |
| BMI group ≥30 (yes) | 1.14 (0.87–1.49) | 0.346 | 1.12 (0.79–1.59) | 0.514 |
| Gender (woman vs. man) | 1.07 (0.82–1.40) | 0.609 | 1 (0.71–1.40) | 0.995 |
| Hypoglycemia group (yes) | 1.75 (1.18–2.59) | 0.005 | 1.45 (0.88–2.39) | 0.146 |
| Central obesity (yes) | 1.15 (0.86–1.53) | 0.352 | 0.98 (0.62–1.53) | 0.923 |
| R W/H Group >0.55 (yes) | 1.24 (0.83–1.86) | 0.300 | 1.34 (0.77–2.36) | 0.303 |
| Barthel Group (severe-total) | 0.004 | 0.622 | ||
| Moderate | 1.70 (0.61–4.78) | 0.311 | 1.77 (0.56–5.66) | 0.332 |
| Independent | 0.61 (0.27–1.40) | 0.242 | 1.40 (0.54–3.65) | 0.489 |
| Charlson group (absence) | 0.000 | 0.000 | ||
| Low comorbidity | 1.91 (0.68–1.51) | 0.947 | 0.94 (0.60–1.47) | 0.778 |
| High comorbidity | 3.6 (2.58–5.00) | 0.000 | 3.36 (2.20–5.12) | 0.000 |
| Macrovascular Dis (yes) | 1.69 (1.26–2.26) | 0.000 | 0.88 (0.59–1.32) | 0.547 |
| Microvascular Dis (yes) | 1.38 (0.96–1.95) | 0.068 | 0.75 (0.48–1.15) | 0.187 |
| Treatments | ||||
| Antiaggregants | 1.24 (0.94–1.63) | 0.121 | 1.08 (0.75–1.55) | 0.676 |
| Anticoagulants | 1.97 (1.35–2.88) | 0.000 | 1.51 (0.96–2.37) | 0.078 |
| Anti-HTN | 2.95 (1.89–4.59) | 0.000 | 2.43 (1.48–4.02) | 0.000 |
| OAD (hypoglycemic agents) | 0.60 (0.42–0.84) | 0.003 | 0.80 (0.53–1.21) | 0.298 |
| Insulin | 1.80 (1.33–2.43) | 0.000 | 1.13 (0.72–1.75) | 0.598 |
OAD: oral antidiabetics; DM2: diabetes mellitus type 2; Dis: disease; KD: kidney disease; HbA1c: glycosylated hemoglobin; HTN: arterial hypertension; CI: confidence interval; BMI: body mass index; OR: odds ratio; R W/H: waist circumference/height.
The objective of the treatment of diabetes in the elderly is to maintain their functional capacity and quality of life, and prevent complications. However, this type of patients not only have to face their disease, but also additional burdens related to the process of aging, associated comorbidities and a higher percentage of geriatric syndromes. It has been evidenced that elderly patients with diabetes have a high prevalence of disability and functional deterioration, which produces a significant impact on the treatment and management of their diabetes.19,20
DM2 and chronic kidney disease are chronic conditions that often coexist in people older than 65 years, in which hypoglycaemic episodes and pharmacological adverse events, that may worsen their quality of life, must be avoided.
Decision making in this group of patients is complex; aspects other than biomedical objectives must be addressed, including morbidity and life expectancy. In this regard, it is remarkable the low level of HbA1c achieved in the patients from our study, 7.04% of patients, with a mean age of 76.4 years, and a 88.9% of patients with values lower than 8,5%, values that are similar to those obtained in other studies conducted in our environment,4,12,21–23 with HbA1c between 6.84 and 7.3%, mean ages between 66.7 and 68.8 years and a 80–88% of patients with HbA1c<8.5%. In studies from other Western countries (United States, Finland and France)8,9,24 in patients with average ages between 60.5 and 71.2 years, the mean HbA1c values were between 7.03 and 7.2%, Similar the values observed in our study, which may suggest a possible overtreatment of this type of patients, which together with the deterioration of their renal function may favor hypoglycemic episodes.
In the studied population, attended by PC physicians and with an average of 11.6 years evolution of their DM, the prevalence of CKD was 37.2%, of RI (eGFR<60mL/m/1.73m2) was 29.7% and with albuminuria 20.6% (moderately high 17.8%, severely high 2.8%). These results show a prevalence of CKD which is higher than in recent studies in our country. In the Percedime study212 performed by PC in outpatients clinics in a diabetic population >40 years of age, mean age of 66.8 years and an average of 9 years of evolution of their DM2, the reported prevalence of CKD was 27.9%, 18% had RI and albuminuria was present in 15.4% (moderately high 13%, severely increased 3.2%); unlike our study and others performed in Spain and in other countries, several analytical determinations were made to confirm the results. Previous work done in the province of Teruel25 in a diabetic population over 18 years of age, mean age 67.9 years and with a single analytical determination, showed a prevalence of CKD in 34.6%, RI in 25.2% and albuminuria in 16.1% (moderately high 14.3%, severely increased 1.8%). Also in our country, Vinagre et al.23 reported in diabetics over 30 years of age, mean age of 68.2 years and average duration of their DM2 of 6.5 years, a prevalence of RI in 20% and albuminuria in 16.7% of patient (moderately high in 14.9%, severely elevated in 1.8%). In the study by Coll et al.4 in patients with DM2 with a mean age of 68 years, mean duration of their diabetes 7 years and with a single determination of albuminuria, the prevalence of CKD was 34.1%, RI in 22.9% and albuminuria in 19.5%, values that are similar to the obtained in our study. In Finland, in a study conducted in PC centers9 in diabetics over 29 years of age, mean age 67 years, average duration of their diabetes 9.2 years and with a single determination of ACR, the prevalence of CKD was 34.7%, RI in 16.2% and albuminuria in 24.3% (moderately high 17.1%, severely elevated 7.2%), it should be stressed the percent of severely elevated albuminuria, which double or triple those observed in Spanish studies. Another study conducted in PC in Switzerland,10 in patients with an average age of 66.5 years and an average duration of DM2 of 9.3 years, showed a prevalence of CKD of 45.4% and RI of 22.4%; only 30% of the patients contributed one determination of albuminuria. The study by Wu et al.,8 using data from the US National Health and Nutrition Examination Surveys 2007–2012, with a single analytical determination, reported a prevalence of CKD of 38.3% in diabetics over 18 years of age with an average duration of DM2 of 10 years; the prevalence of RI was 19.8%. Considering only patients older than 65 years, the prevalence of CKD was 58.7% and RI 40.8%, which are almost double than in our study. The different prevalences reported by separate studies, may be due to methodological differences, a single analytical determination or several to confirm the results, or to the ethnic and demographic characteristics of the population.
Early detection of CKD has important clinical implications since both albuminuria and decreased glomerular filtration are independently associated with cardiovascular morbidity and mortality as well as total mortality26; thus, it may condition the therapeutic options of DM2 and other comorbidities present in this type of patients. In our study, 82.3% of patients used some type of oral antidiabetics and 25.2% were treated with insulin. Metformin is the OAD most used, in 70.2% of patients and only 0.8% used Metformin outside the current indication of the European Medicines Agency27 (stages G4–G5 of ERC) and the Spanish Consensus Documents on detection17 and treatment of DM2 in the patient with CKD,28 even though in the technical data sheet of metformin it is contraindicated if eGFR<60mL/m/1.73m2. Sulfonylurea (SU) was used by 16.3% of patients (2.6% in stages G4–G5 out of indication).17,28 In the study by Ruiz Tamayo et al.,29 88.4% used metformin, and in 36.4% of the cases it was out of formal indication; SU was used by 31.1% of the patients, in 66% of the cases the used was out of indication. This important discrepancy may be due to the involvement of the medical researchers in the follow-up of their diabetic patients (in our study) or to the lack of registration of the prescription. Muller et al.24 reported use of metformin use in 45.2% of patients (49.3% out of indication), 32.2% used SU (26.7% out of indication) and 34.5% were on insulin, with a progressive increase in its use according to the deterioration of renal function. In another French study,30 86% of patients used metformin (33% out of indication), 32% were on SU (20% off-label cases) and 19% in patients on insulin (61% in more severe stages of CKD).). In the Swiss study,10 74% used metformin (43.8% off-label), 20.5% used SU (9.4% off-label) and 28.9% used insulin (50% in CKD Stages G4,G5). In the study by Wu et al.,8 55.6% of diabetics used metformin (6.5% in CKD stages G4–G5), a 35.4% used SU (in more than 50% of the case out of indication) and 18.9% used insulin (38% in stages G4–G5 of ER). This study also reports the use of inhibitors of the renin–angiotensin–aldosterone system for the treatment of hypertension in 62% of patients (in our study it reach almost 70%) and the use of thiazide diuretics in 23.7% of diabetic hypertensive patients (33.4% in stage G4 of CKD), while in our study, 38.7% of hypertensive diabetics used this group of drugs, only 1.7% with eGFR<30mL/m/1.73m2 situation in which its use is not recommended due to lack of a significant effect. In short, although the various studies tend to reflect, in their prescription pattern, the recommendations of the guidelines for the treatment of individuals with CKD,31 the number of patients using some OADs outside of the indication is strikingly high.
In our study, 7% of the patients presented a moderate-total degree of dependence and 42.8% had a high comorbidity, both entities being related to the deterioration of renal function. A 56.1% of patients with moderate dependence and 34.8% with severe dependence have CKD, 17.7% of patients with average comorbidity and 46.1% of those with high comorbidity have RI. It should be noted that in the study REAnal Disease Morbidity in diabetic and non-diabetic patients (MERENA),32,33 launched by the Spanish Study Group of Diabetic Nephropathy, the baseline study data corresponding to 1129 patients with CKD stages 3 and 4, show that the cohort of diabetic patients (40.8% of the total, 91.7% DM2) has greater cardiovascular morbidity than the non-diabetic population. In the study by Kim et al.,34 conducted in Korea, patients had an average Charlson Index of 3.3 (2.7 in our study) and 60.8% of the patients had a high comorbidity (Charlson ≥3) that was associated with twice the risk of hypoglycemic episodes.
The multivariate analysis supports previous research that identifies advanced age, treated HTN and the presence of high comorbidity as potential factors of CKD in the diabetic population; contrary to other reports, we did not find an association with the duration of DM, HbA1c and gender.4,8,12
The strengths of our work are: first, it includes characteristics of a group of patients underrepresented in clinical trials. Second, it measures the degree of dependence and comorbidity of the participants.
The study has several limitations: albuminuria was only provided by 65.5% of the patients and only one analytical determination was collected, which is usual in epidemiological studies but does not allow to distinguish between a transient and persistent alteration. Therefore, the estimates of CKD reported in this work may have been higher than if the eGFR and ACR measures were repeated to meet the KDIGO16 criteria and the Spanish consensus document,17 to demonstrate persistence of alterations for 3 or more months for diagnosis of chronic kidney disease. The availability of CAC values in all participants could reduce the possible overestimation, in fact if only patients who contributed with albuminuria were considered, the prevalence of CKD was 39.5%. The race was not taken into account in the calculation of the eGFR, a situation that is not very relevant given the homogeneous characteristics of the population studied.
ConclusionsChronic Kidney Disease is common in the elderly diabetic population and it is associated with a high frequency of concomitant chronic diseases and a greater degree of dependence. The use of metformin and sulfonylureas in advanced stages of Renal insufficiency is scarce but should be adjusted or eliminated in each case, since the use may by inadequate. Insist on the need to determine the ACR in urine as a necessary tool for the detection of CKD, since these patients with diabetes and CKD require careful attention and monitoring. They have a high risk of cardiovascular disease and suffer side effects from medications. However, it is likely that these problems are usually underestimated in clinical practice, so greater emphasis should be placed on alerting professionals to act in these situations.
FundingTo perform this study, we had a collaboration grant between the Spanish Diabetes Society and Boehringer-Ingelgeim. Financial sources had no role in the design of the study, collection or interpretation of the data and have not participated in the preparation, revision or approval of the manuscript.
Conflict of interestsThe authors declare that they have no conflicts of interest.
Daniel Bordonaba Bosque, from the Aragonese Institute of Health Sciences, for the statistical treatment of the data.
To the researchers of the ESCADIANE study in Spain:
Andalusia: Acosta González MD, Ávila Lachica L, Cano García MS, Carretero Anibarro E, Cobo Burgos S, Fábrega Escolá G, Fernández Baena M, García Lozano MJ, Garrido Redondo N, Ginel Mendoza L, Gómez García MC, Iranzo Luna AM, Losada Ruiz C, Martín Montero G, Pérez Verdugo J, Requena Carrión E, Ruiz Serrano M, Velázquez Lupiáñez L, Vergara Martín J.
Aragón: Ariza Ortín R, Canta Castro L, Chicote Abadía B, Gil Orna P, Gracia Tricas MM, Trillo JM Millaruelo, Rascón Velázquez MS, Sangrós González FJ, Sanz Rebollo G, Torrecilla Conde J.
Balearic Islands: Angullo Martínez E, Segui Díaz M.
Canary Islands: Álvarez Hernández SM, Carrillo Fernández L, Fernández JA.
Cantabria: Arnaiz de las Revillas JM.
Castilla León: Gamarra Ortiz J, Gutiérrez Almarza MA, Sánchez Cabrero LG.
Castilla La Mancha: Gómez González L.
Catalonia: Barrot de la Puente J, Benito Badorrey B, Bobe Molina I, Cuatrecasas Cambra G, Franch Nadal J, Hidalgo Ortiz M, Luna Redondo G, Mata Cases M, Mur Martí T, Prats de la Iglesia P, Rodero Nuño M, Ruiz Tamayo I.
Extremadura: Carramiñana Barrera F, Same Friar D.
Galicia: García Soidán FJ, Malo García F, Martínez Vidal A, Navarro Echeverría MA.
La Rioja: Babace Istúriz C, Torres Baile JL.
Madrid: Artola Menéndez S, Bedoya Frutos MJ, García Caro MG, Ibañez Brillas M, López Palomar R, Nogales Aguado P, Rollán Landeras MT, Sala Arnaiz C, Serrano Martín R, Yanes Baonza M.
Murcia: Álvarez García B, Granero Fernández E, Hernández Menarguez F, Martínez Candela J.
Navarra: Astrain Jaunsaras L, Buil Cosiales P, Díez Espino J, Escriche Erviti L, Fernández Clavero E.
Basque Country: Ezcurra Loiola P.
Valencia: Marco Macián MD, Salanova Peñalba A.
Please cite this article as: Martínez Candela J, Sangrós González J, García Soidán FJ, Millaruelo Trillo JM, Díez Espino J, Bordonaba Bosque D, et al. Enfermedad renal crónica en España: prevalencia y factores relacionados en personas con diabetes mellitus mayores de 64 años. Nefrologia. 2018;38:395–407.