Evidence for the efficacy and safety of oral anticoagulation with dicumarines in patients with atrial fibrillation (AF) on hemodialysis is controversial. The aim of our study is to evaluate the long-term prognostic implications of anticoagulation with dicumarines in a cohort of patients with non-valvular AF on a hemodialysis program due to end-stage renal disease.
MethodsRetrospective, observational study with consecutive inclusion of 74 patients with AF on hemodialysis. The inclusion period was from January 2005 to October 2016. The primary variables were all-cause mortality, non-scheduled readmissions and bleeding during follow-up.
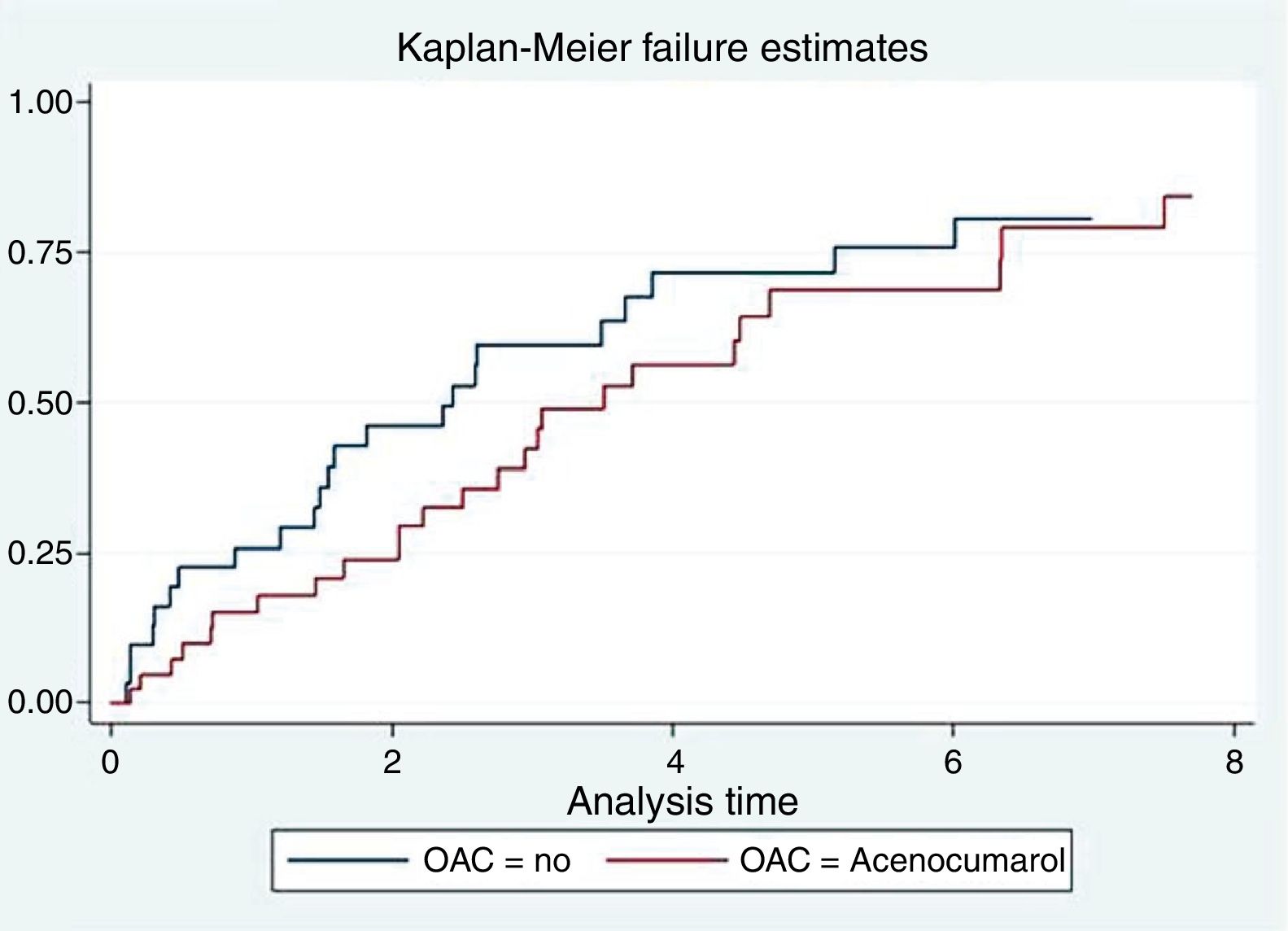
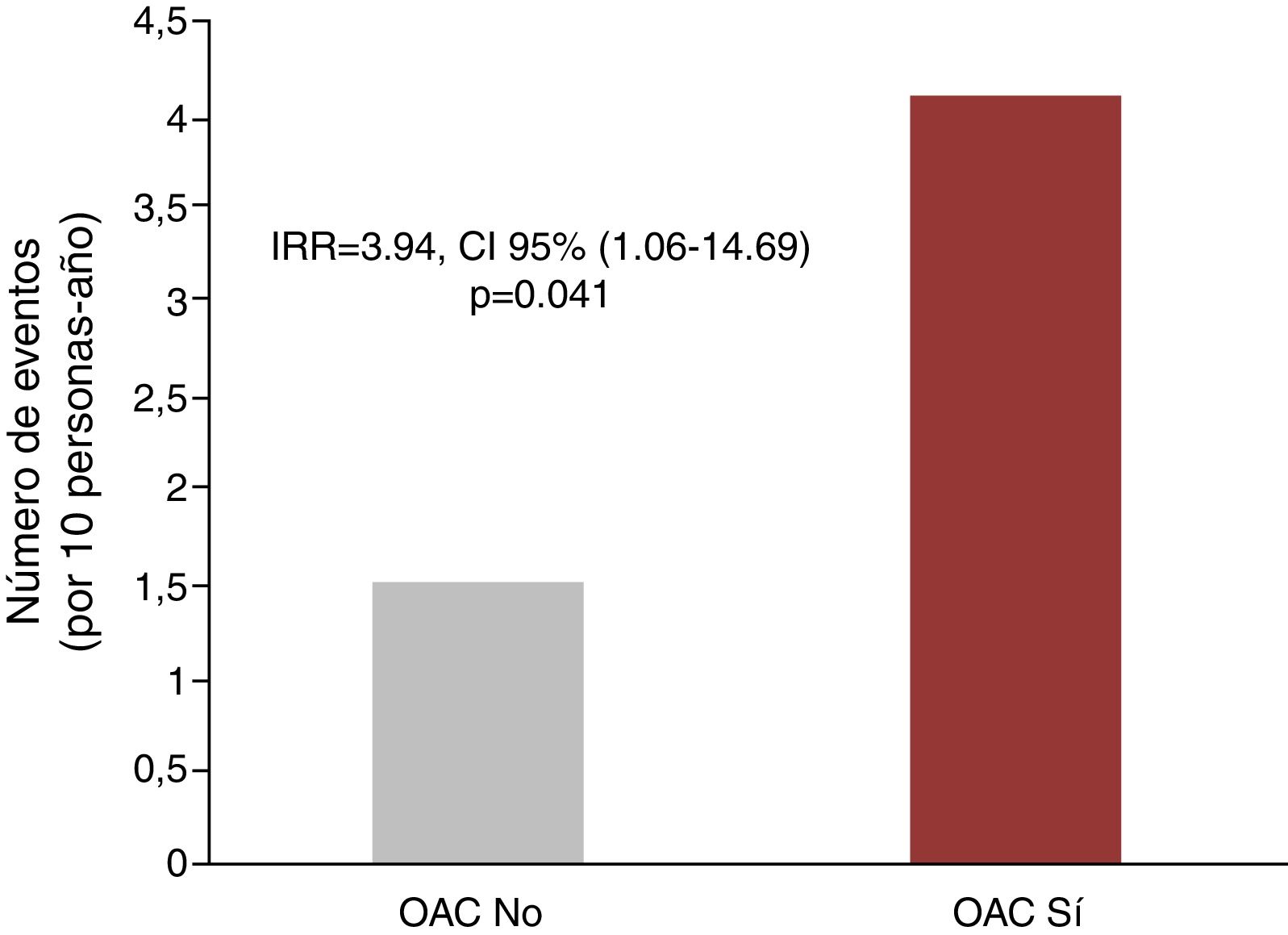
ResultsMean age was 75±10 years; 66.2% were men and 43 patients (58.1%) received acenocoumarol. During a median follow-up of 2.40 years (IQR=0.88–4.15), acenocoumarol showed no survival benefit [HR=0.76, 95% CI (0.35–1.66), p=0.494]. However, anticoagulated patients were at increased risk of recurrent cardiovascular hospitalizations [IRR=3.94, 95% CI (1.06–14.69), p=0.041]. There was a trend toward an increase in repeated hospitalizations of ischemic cause in anticoagulated patients [IRR=5.80, 95% CI (0.86–39.0), p=0.071]. There was a statistical trend toward a higher risk of recurrent total bleeding in patients treated with acenocoumarol [IRR=4.43, 95% CI (0.94–20.81), p=0.059].
ConclusionsIn this study, oral anticoagulation with acenocoumarol in patients with AF on hemodialysis did not increase survival. However, it was associated with an increased risk of hospitalizations of cardiovascular causes and a tendency to an increased risk of total bleeding.
La evidencia de la eficacia y seguridad de la anticoagulación oral con dicumarínicos en pacientes en hemodiálisis con fibrilación auricular (FA) es controvertida. El objetivo de nuestro estudio es evaluar las implicaciones a nivel pronóstico a largo plazo de la anticoagulación con dicumarínicos en una cohorte de pacientes con FA no valvular en programa de hemodiálisis debido a insuficiencia renal terminal.
MétodosEstudio observacional retrospectivo con inclusión consecutiva de 74 pacientes en hemodiálisis con FA. El periodo de inclusión fue de enero de 2005 a octubre de 2016. Las variables principales fueron mortalidad por todas las causas, reingresos no programados y sangrados.
ResultadosLa edad media fue de 75±10años; el 66,2% fueron hombres y 43 pacientes (58,1%) recibieron acenocumarol. Durante una mediana de seguimiento de 2,40años (IQR=0,88-4,15), el acenocumarol no demostró beneficio en supervivencia [HR=0,76, IC 95% (0,35-1,66), p=0,494]. Sin embargo, los pacientes anticoagulados presentaron más riesgo de hospitalizaciones cardiovasculares recurrentes [IRR=3,94, IC 95% (1,06-14,69), p=0,041]. Hubo una tendencia a un aumento de hospitalizaciones repetidas de causa isquémica en los pacientes anticoagulados [IRR=5,80, IC 95% (0,86-39,0), p=0,071]. Se observó una tendencia estadística hacia un mayor riesgo de sangrados totales recurrentes en los anticoagulados [IRR=4,43, IC 95% (0,94-20,81), p=0,059].
ConclusionesEn el presente estudio, la anticoagulación oral con acenocumarol en pacientes en hemodiálisis con FA no supuso un aumento de la supervivencia, y sin embargo, se asoció con un mayor riesgo de hospitalizaciones de causa cardiovascular y una tendencia a mayor riesgo de sangrados totales.
Patients on hemodialysis show a higher prevalence of atrial fibrillation (AF) than the general population.1 The CHA2DS2-VASc score has been widely validated to guide on the need for anticoagulation therapy for prevention of thromboembolic events in non-valvular AF in the general population.2 Due to the prevalence of heart failure, hypertension and diabetes mellitus, most patients on hemodialysis with AF would require oral anticoagulation based on CHA2DS2-VASc scale. The efficacy and safety of oral anticoagulation with dicoumarin in hemodialysis patients with AF is controversial.3–5
In the present study, we intend to evaluate the long-term prognostic implications of anticoagulation with antivitamin K drugs in a cohort of patients on maintenance of hemodialysis with non-valvular AF.
MethodsPatient population and Study designThis is a retrospective observational study. From January 2005 to October 2016 all patients from our center hemodialysis program with AF were added consecutively to the study. Patients with mechanical prostheses and significant mitral stenosis were excluded. A total of 74 hemodialysis patients with AF were included. The decision to treat with oral anticoagulation was based on the medical criteria of the nephrologist and/or cardiologist responsible for the care of each patient. The anticoagulant drug administered was acenocoumarol in all cases.
Data collectionThe clinical information was obtained from the electronic medical records of the Nephrology and Cardiology departments containing all data from outpatient and hospital care are registered. Additional information was extracted from the Hospital Discharge Program, provided by the Regional Health Administration mainly containing data of emergency care episodes and hospitalizations in other units. In anticoagulated patients, the international normalized ratios (INR) and the therapeutic range times (TRT) were recorded during the follow-up period; this was accomplished by analyzing the electronic database of the Hematology Service.
Objectives and definitionsThe primary objectives were: all cause mortality, unscheduled re-admissions (due to specific or non-specific causes) and bleeding, during follow-up. Hospitalization of a cardiovascular cause was defined as admissions due to acute coronary syndrome, heart failure, stroke, bleeding and peripheral ischemia. Hospitalizations due to ischemia were hospital admissions due to cardiac, cerebral or peripheral ischemia. Ischemic stroke was defined as a neurological deficit of recent onset that persisted 24h and was confirmed by imaging techniques such as computed tomography or magnetic resonance imaging. Major hemorrhages were considered in those cases of fatal bleeding (with fatal outcome) and those requiring transfusion and/or hospitalization. The follow-up was censored in the event of death or interruption of anticoagulant treatment during the follow-up (n = 8).
Statistical analysisContinuous variables are presented as mean ± standard deviation or median [interquartile range (IQR)]. Discrete variables are shown as percentages. A propensity index (PS) was generated to minimize potential confounding biases. Those variables associated with the probability of receiving anticoagulation with acenocoumarol (p<0.25 in univariate analysis) were included in a logistic regression model, creating a final simplified model using a “backward” covariable selection algorithm. The final model included a set of 12 covariates: gender, AF/paroxysmal flutter (yes/no), ischemic heart disease (yes/no), chronic obstructive pulmonary disease (yes/no), peripheral artery disease (yes/no), previous stroke (yes/no), valvular disease (yes/no), left ventricular function (%), treatment with antiaggregants, treatment with digoxin, treatment with antiarrhythmics, treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists. The area under the receiver operating curve (ROC) showed an excellent discriminative capacity (0.936) to predict the probability of receiving anticoagulation. The CHA2DS2-VASc and HASBLED scores were not independently associated with the probability of receiving acenocoumarol.
To explore the prognostic effect of acenocoumarol in our study population, we performed a multivariate Cox regression model and a negative binomial regression (NBreg) including the quartiles of PS and the CHA2DS2-VASc score for death and recurrent hospitalizations, respectively.
For recurrent hemorrhagic events, a negative binomial regression was used and the risk estimates were adjusted by quartiles of the PS and HASBLED score. The risk estimation is shown as hazard ratios (HRs) for death and incidence rate ratios (IRRs) for recurring events. All analyses were performed using Stata 14.1.
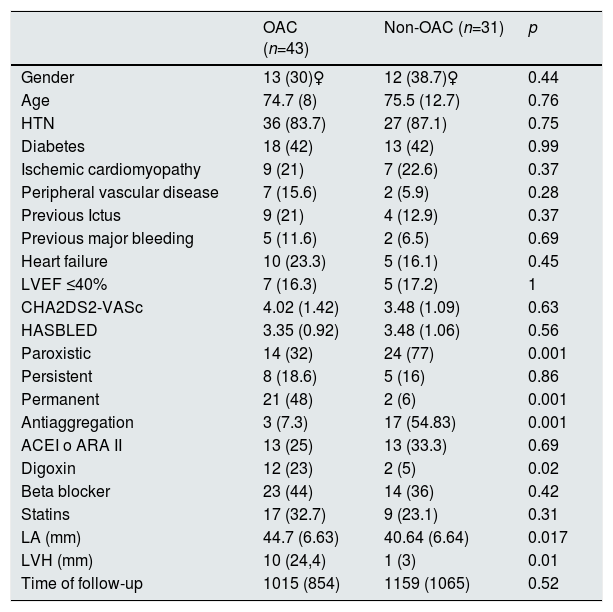
ResultsThe patients mean age was 75±10 years; 66.2% were men and 43 patients (58.1%) received acenocoumarol. The estimated median (IQR) of glomerular filtration rate, CHA2DS2-VASc and HASBLED was 7.3 (5.4–10.2)mL/min/1.73m2, 43–5 and 3,3,4 respectively. Table 1 shows the baseline characteristics of the patient population.
Baseline characteristics of hemodialysis patients with non-valvular atrial fibrillation and atrial flutter. Anticoagulated versus those not anticoagulated.
| OAC (n=43) | Non-OAC (n=31) | p | |
|---|---|---|---|
| Gender | 13 (30)♀ | 12 (38.7)♀ | 0.44 |
| Age | 74.7 (8) | 75.5 (12.7) | 0.76 |
| HTN | 36 (83.7) | 27 (87.1) | 0.75 |
| Diabetes | 18 (42) | 13 (42) | 0.99 |
| Ischemic cardiomyopathy | 9 (21) | 7 (22.6) | 0.37 |
| Peripheral vascular disease | 7 (15.6) | 2 (5.9) | 0.28 |
| Previous Ictus | 9 (21) | 4 (12.9) | 0.37 |
| Previous major bleeding | 5 (11.6) | 2 (6.5) | 0.69 |
| Heart failure | 10 (23.3) | 5 (16.1) | 0.45 |
| LVEF ≤40% | 7 (16.3) | 5 (17.2) | 1 |
| CHA2DS2-VASc | 4.02 (1.42) | 3.48 (1.09) | 0.63 |
| HASBLED | 3.35 (0.92) | 3.48 (1.06) | 0.56 |
| Paroxistic | 14 (32) | 24 (77) | 0.001 |
| Persistent | 8 (18.6) | 5 (16) | 0.86 |
| Permanent | 21 (48) | 2 (6) | 0.001 |
| Antiaggregation | 3 (7.3) | 17 (54.83) | 0.001 |
| ACEI o ARA II | 13 (25) | 13 (33.3) | 0.69 |
| Digoxin | 12 (23) | 2 (5) | 0.02 |
| Beta blocker | 23 (44) | 14 (36) | 0.42 |
| Statins | 17 (32.7) | 9 (23.1) | 0.31 |
| LA (mm) | 44.7 (6.63) | 40.64 (6.64) | 0.017 |
| LVH (mm) | 10 (24,4) | 1 (3) | 0.01 |
| Time of follow-up | 1015 (854) | 1159 (1065) | 0.52 |
Continuous variables are expressed as mean ± standard deviation. Discrete variables are expressed as a numeric value of %. OAC: oral anticoagulation; LA: left atrium; ARA II: angiotensin II receptor antagonists; LVEF: left ventricular ejection fraction; HTN: arterial hypertension; LVH: left ventricular hypertrophy; ACE inhibitors: angiotensin-converting enzyme inhibitors.
In the anticoagulated patients, the AF was permanent to a large extent (48% vs 6%, p<0.001), the left atria was more dilated (44.7 ± 6.6mm vs 40.7±6.6mm, p=0.017) and a higher proportion of a significant left ventricular hypertrophy (≥14mm) (24% vs 2.9%, p=0.01). Regarding the treatment, the proportion of patients on antiplatelet therapy was greater in non-anticoagulated than anticoagulated patients (54.8% vs 7.3%, p=0.000). Conversely, digoxin treatment was more frequent in anticoagulated patients (23% vs 3%, p=0.02). No other differences were observed with respect to the rest of the baseline characteristics. Anticoagulated patients presented a median TRT of 33.3% (IQR=15–48).
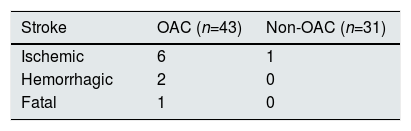
Anticoagulation and adverse clinical eventsDuring a median follow-up of 2.4 years (IQR = 0.88–4.15), 51 (68.9%) patients died, there were 125 hospitalizations due to all causes in 53 patients (71.6%), 65 hospitalizations due to cardiovascular causes in 32 patients (43.2%), 16 hospitalizations due to stroke or bleeding in 14 patients (18.9%) and 26 hospitalizations of ischemic causes in 18 patients (24.3%). There were nine strokes (two hemorrhagic and seven ischemic), all were in the group of anticoagulated patients except one ischemic stroke in a patient without anticoagulation; only one of them had a fatal outcome (it was an hemorrhagic stroke in an anticoagulated patient) (Table 2).
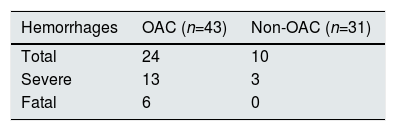
Regarding the episodes of bleeding, there were 34 hemorrhages in 24 patients (32.4%) and 16 were major bleedings in 13 patients (17.6%). The anticoagulated patients presented 24 bleedings, of which 13 were severe and 6 fatal. The group of patients without anticoagulation had 10 hemorrhages, of which 3 were severe, none was fatal (Table 3).
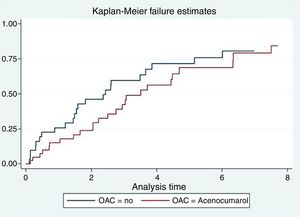
Anticoagulation and mortalityIn the univariate analysis, anticoagulation with acenocoumarol was not associated with lower mortality (2.78 vs 2.01 per 10 person-year, p=0.304), as represented by the Kaplan–Meier curve (Fig. 1). In the multivariate analysis adjusting for PS and CHA2DS2-VASc, anticoagulation had a neutral effect on survival [HR = 1, 0.76, 95% CI (0.35–1.66), p=0.494)].
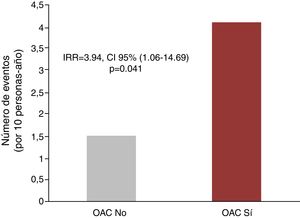
Anticoagulation and recurrent hospitalizationsIn the univariate analysis, anticoagulation was associated with a higher rate of recurrent hospitalizations due to all causes (6.75 vs 4.39 per 10 person-year, p=0.202), due to cardiovascular causes (4.10 vs 1.50 for 10 persons-year, p<0.001) and hospitalizations due to ischemic causes (1.77 vs 0.43 for 10 persons-year; p=0.003), as shown in Fig. 2. In the multivariate analysis, and after adjusting for PS and CHA2DS2-VASc score, the differences for hospitalization of all causes did not reach statistical significance [IRR = 2.13, 95% CI (0.78–5.86), p=0.141]. However, for recurrent cardiovascular
hospitalizations, the excess risk attributable to anticoagulant therapy remained significant after the multivariate adjustment [IRR = 3.94 CI, 95% CI (1.06–14.69), p=0.041]. Finally, there was also a tendency to an increased risk of repeated hospitalizations of ischemic cause in anticoagulated patients [IRR = 5.80, 95% CI (0.86–39.0), p=0.071].
Anticoagulation and recurrent bleedingThere was a tendency of a higher frequency of total hemorrhages and also major hemorrhages in anticoagulated patients [1.93 vs 1.11 (p=0.113) and 1.05 vs 0.32 (p=0.051)]. After multivariate adjustment, we observed a tendency toward an increased risk of recurrent bleeding in the anticoagulated patients [IRR = 4.43, 95% CI (0.94–20.81), p=0.059]. Regarding major bleeding, although the differences were ostensible, these did not become statistically significant [IRR= P13.38, 95% CI (0.47–382.68), p<0.129)].
Time in therapeutic range (TRT) and hemorrhagic events in anticoagulated patientsOur anticoagulated patients presented a median TRT of 33.3% (IQR = P15-48).
The anticoagulated patients with major bleeding had a higher percentage of INR controls above the therapeutic range than the anticoagulated patients without major hemorrhages; the respective medians were 19% (IQR = 2–26%) vs 10% (IQR = 0–26%), p<0.01.
DiscussionThe benefit of oral anticoagulation in patients with AF has been demonstrated in randomized studies.2 In these trials, end-stage renal failure has been systematically an exclusion criterion. There is no evidence that this benefit can be extrapolated to hemodialysis patients6–8; and, it is well known that the risk of ischemic and hemorrhagic complications in these patients is higher than that of the general population.2,4,6,9
In our study, which evaluated a group of patients with AF and end-stage renal disease broadly representative of daily clinical practice, it was observed that oral anticoagulation did not present any benefit in terms of survival. Our results are in agreement with a meta-analysis that included 37.349 patients of similar characteristics, in which anticoagulation did not show any benefit in mortality.4,10–15
Regarding recurrent hospitalizations, the present study showed an increase in recurrent hospitalizations of cardiovascular causes, with a tendency to increase the risk of hospitalizations due to ischemic causes. These findings are of special relevance given that previous studies in hemodialysis patients with AF receiving anticoagulation did not analyze recurrent hospitalizations.
With respect to the risk of bleeding, in the present study we found a significant increase in the risk of recurrent bleeding, which, however, did not become statistically significant, probably because of the small sample size. Consistent with these results, four recent meta-analysis of observational studies suggest that warfarin should not be used routinely in patients on hemodialysis with AF since it does not provide a benefit in mortality and strokes are not prevented significantly however it does increase the risk of hemorrhage in these patients.5,16–18
Our study population had a high thromboembolic risk as determined by the CHA2DS2-VASc scale, with a median of 4 (IQR = 3–5), so oral anticoagulation should be recommended. To date, there is not enough evidence supporting anticoagulation in these patients based on this risk scale. Furthermore, renal failure has been shown to be an independent risk factor for stroke.19,20 The mechanism of stroke in patients on hemodialysis with AF is not clearly established. It is likely that in these patients there is a greater risk of atherothrombotic than embolic stroke.5 And, there is evidence that warfarin may be associated with an increased risk of calciphylaxis21,22 and accelerated vascular calcification in hemodialysis patients.23,24
The antiaggregant treatment has not been shown to be effective in reducing strokes of cardioembolic origin or strokes due to systemic embolisms,25 however it is effective in preventing atherothrombotic events, so it could have contributed to the reduction of ischemic events of this origin in the population of non-anticoagulated patients, given that many of them were on antiplatelet therapy (54.8% vs 7.3%, p=0.000).
Patients who are anticoagulated in hemodialysis frequently present labile INR (TRT below 60%), as reported in the few studies in which this parameter has been analyzed.4,13,26 In this line, the recent study by Szummer et al.27 shows that TRT is lower in patients with chronic kidney disease, and a low TRT is associated to poor prognosis independently of renal function. In fact, our patients had a median TRT of 33.3% (IQR = P15–48). This finding could, in some way, be related to the poor clinical results of anticoagulation with antivitamin K in these patients. In the study of Limdi et al.28, advanced renal failure is associated with an increase in the frequency of supra-therapeutic ranges of INR and an increased risk of bleeding; in fact, in our study, anticoagulated patients who presented major bleeding had a higher percentage of INR controls above the therapeutic range than anticoagulated patients without major hemorrhages, with a median of 19% (IQR=2–26%). compared to 10% (IQR=0–26%), p<0.01.
Unfortunately, we are unable to perform an analysis of those who had a TRT greater than or equal to 60% compared to the rest since there were only two patients showing optimal control, so it is not a sufficient number to establish a comparison.
Despite the inherent limitations due to the sample size, and given that in a traditional methodological approach from time to the first event, the recommendations suggest to have a minimum of 10 events for each covariate included in the multivariate model29 it was decided to use an adjustment through a PS, with the intention to generate a model with excellent discriminative power, generally above 0.85. In our case, the discriminative capacity of the model was >0.90, which indicates an excellent discriminative power. The adjustment made here using a PS (to try to minimize the residual confounding biases typical of observational studies) is in line with what is traditionally accepted.
In an attempt to increase the power of the study and to better describe the natural history of the disease, we decided to explore the relationship between anticoagulation and repeated events (all events) throughout the follow-up. With this approach, which in recent years has been used an promoted by important research groups,30–32 we have recorded 125 hospitalizations for all causes and 65 hospitalizations of cardiovascular causes, which undoubtedly increase the power of the study and offer a more solid risk estimates.
LimitationsThe limitations of our study are those inherent to a retrospective, observational analysis in a single center; however, the population evaluated here is a reflection of the everyday clinical practice that includes all patients with AF in hemodialysis during a very long period, from January 2005 to October 2016, and with a prolonged follow-up [median follow-up of 2.4 years (IQR=0.88–4.15)]. In addition, this is a small study with limited power to detect significant differences, especially if there is a reduced number of adverse episodes. To minimize confounding biases we use an adjustment through a PS. To increase the power of the study, we performed an analysis including all the repeated events throughout the follow-up. In the present study it has not been possible to perform an event analysis in patients with optimal TRT since only two of the anticoagulated patients fulfilled this condition, which highlights the difficulty in controlling anticoagulation with dicoumarin in these patients.
ConclusionsIn the present study, oral anticoagulation with acenocoumarol in patients on hemodialysis with AF did not increase survival, and it was associated with an increased risk of hospitalizations due to cardiovascular causes and there was a tendency to increase the risk of total bleeding. New studies, preferably prospective and in more controlled scenarios are necessary to reveal the usefulness of anticoagulation in these patients..
Conflict of interestThe authors declare to have no conflicts of interest.
Please cite this article as: Soriano RM, Molinab MD, Fernándeza CI, Juliá-Sanchísc R, López Mencherob R, Fernándezb CP, et al. Impacto pronóstico a largo plazo de la anticoagulación en pacientes en hemodiálisis con fibrilación auricular. Nefrologia. 2018;38:388–394.