Left ventricular diastolic dysfunction (LVDD) is an independent predictor of mortality in Chronic Kidney Disease (CKD). The increase in the E/E′ ratio is an indicator of LVDD. The association between cardiovascular risk factors (CVRFs) and E/E′ in children with automated peritoneal dialysis (APD) has not been widely studied.
ObjectiveTo measure the association between CVRFs and E/E′ in children with CKD on APD.
MethodsCross-sectional, prolective, observational, analytical study of children aged 6–16 years on APD. We recorded age, gender, time since onset, time on dialysis, and measured weight, height, blood pressure, haemoglobin, albumin, calcium, phosphorus, parathyroid hormone, and C-reactive protein. E/E′ ratio was measured and considered to have increased when it was higher than 15.
ResultsTwenty-nine children were studied, (19 females). Age was 14.0±2.5 years, and 16.9±11.2 months with substitutive therapy. One patient had reduced left ventricular ejection fraction, and 21 (72.4%) had increased E/E′. E/E′ correlated significantly with haemoglobin (r=–0.53, p=0.003). Haemoglobin and albumin were significantly lower (9.72±1.9 vs. 12.2±1.8; p=0.004 and 3.6±0.5 vs. 4.0±0.3; p=0.035) and the proportion of patients with anaemia and hypoalbuminemia was significantly higher (85.7% vs. 37.5%; p=0.019 and 61.9% vs. 12.5%; p=0.035) in patients with increased E/E′. Haemoglobin was the only independent predictor of E/E′ (β=–0.66; p=0.020) and patients with anaemia were 10 times more likely to have increased E/E′ (95% CI 1.5–65.6, p=0.016).
Conclusions75% of the children had increased E/E′. Anaemia and hypoalbuminemia were significantly related with an increased E/e’.
La disfunción diastólica del ventrículo izquierdo es predictor independiente de mortalidad en insuficiencia renal crónica (IRC). El incremento de la relación E/e’ es un indicador de disfunción diastólica del ventrículo izquierdo. La asociación entre factores de riesgo cardiovascular y E/e’ en niños con diálisis peritoneal automatizada (DPA) ha sido poco estudiada.
ObjetivoMedir la asociación entre los factores de riesgo cardiovascular y E/e’ en niños con IRC en DPA.
MétodosEstudio transversal, prolectivo, observacional, analítico de niños de 6-16 años en DPA. Medimos la edad, el género, el tiempo de evolución, el tiempo en diálisis, el peso, la talla, la tensión arterial, la hemoglobina, la albúmina, el calcio, el fósforo, la hormona paratiroidea y la proteína C reactiva. Se midió E/e’ y se consideró incrementada cuando fue mayor de 15.
ResultadosEstudiamos 29 niños (19 mujeres) con edad de 14,0±2,5 años y 16,9±11,2 meses en tratamiento sustitutivo. Un paciente tuvo fracción de eyección ventricular izquierda disminuida, 21 (72,4%) relación E/e’ incrementada. E/e’ correlacionó significativamente con hemoglobina (r=–0,53, p=0,003). La hemoglobina y la albúmina fueron significativamente menores (9,72±1,9 vs 12,2±1,8; p=0,004 y 3,6±0,5 vs 4,0±0,3; p=0,035) y la proporción de pacientes con hipoalbuminemia y con anaemia fue significativamente mayor (85,7% vs 37,5%; p=0,019 y 61,9% vs 12,5%; p=0,035) en los pacientes con E/e’ incrementada. La hemoglobina fue el único predictor independiente de E/e’ (β=–0,66; p=0,020). Los pacientes con anaemia tuvieron 10 veces más probabilidad de E/e’ incrementada (IC 95% 1,5-65,6, p=0,016).
ConclusionesEl 75% de los niños tuvieron E/e’ incrementada. La anaemia y la hipoalbuminemia se asociaron significativamente con E/e’ incrementada.
Chronic kidney disease (CKD) is a global public health problem; its incidence and prevalence are increasing, as well as the associated costs for healthcare. At the end of 2014, 9721 children were undergoing treatment for end stage chronic kidney disease in the United States, and the probability of 5-year survival was 90% in within the 2005–2009 period. Cardiovascular disease is the main cause of death in patients with CKD. In its report from 2016, the United States Renal Data System showed that while one-year mortality in younger than 21-years, adjusted for all causes, was 32 cases per 1000 patients at risk, cardiovascular disease was the cause of death in one third of cases, above infections that caused death in one sixth of patients.1
Left ventricular hypertrophy and dysfunction are temporary manifestations of ventricular damage, and are considered consistent independent predictors of morbidity and mortality in patients with CKD. Left ventricular dysfunction may be detected even before a reduction in the left ventricular ejection fraction is observed. Therefore, the left ventricular dysfunction is considered an early manifestation of cardiac dysfunction. Throughout the last decade, the frequency of these disorders has been studied also in children and young adults with CKD.2
Left ventricular diastolic dysfunction (LVDD) is a clinical syndrome in which patients show signs and symptoms of heart failure, normal or almost normal LV ejection fraction and echocardiographic evidence of abnormal left ventricle filling or increased filling pressure. This heart failure with preserved ejection fraction is more common than heart failure with a reduced ejection fraction.3 The Doppler echocardiography images also showed changes in LV filling and compliance early on in the progression of CKD in children, and its prevalence is increased in patients undergoing dialysis treatment.4
The tissue Doppler echocardiography images of the myocardial velocities during early diastole measured in the mitral annulus (also known as E/E′ wave) are highly sensitive in evaluating the myocardial relaxation status.5 Better still, the ratio that results from dividing the mitral flow E wave values obtained by Doppler ultrasound (E wave) by the E′ wave value obtained in the tissue Doppler (ratio also known as E/E′ ratio) is well correlated with the filling pressure, which is also affected prematurely in the initial stages of diastolic ventricular dysfunction. A reduced E′ wave (<9cm/s) or an E/E′ ratio greater than 15 are evidence of this dysfunction.6,7
Known cardiovascular risk factors in adults are age, being male, hypertension, diabetes, dyslipidaemia8,9 and physical inactivity. In patients with CKD, additional risk factors are uraemia, anaemia,10–12 hypervolaemia, hyperparathyroidism, malnutrition, hypoalbuminaemia,13–15 hyperuricaemia16 and calcium phosphate metabolism disorders,15 as well as inflammation and oxidative stress.17–19
Studies that have searched for a link between cardiovascular risk factors along with the presence of LVDD in children are scarce. It is known that children with CKD, and even more so those who have already started dialysis, have higher E/E′ values than healthy controls, being significantly associated with the presence of anaemia, hyperphosphataemia and increased calcium-phosphate product,4 as well as hypertension and altered levels of vitamin D and parathyroid hormone.20
Among the forms of dialysis treatment in children with CKD, automated peritoneal dialysis is best adapted to the physiological characteristics of their peritoneum, so fluid overload is less frequent and less severe. In the Paediatric Nephrology Department of the UMAE, Hospital General Centro Médico La Raza, the most common form of replacement therapy in children with terminal chronic kidney disease is automated peritoneal dialysis. Elimination of fluid overload and its role as a main cardiovascular risk factor in children, allows us to better study the role that other cardiovascular risk factors in left ventricular diastolic function.
ObjectiveTo determine the significance of the association between the various cardiovascular risk factors and the mitral flow E wave ratio with the tissue Doppler E′ wave (E/E′) as a marker of LVDD in children with CKD on automated peritoneal dialysis.
MethodsAfter authorisation from the local research committee we performed this observational, prolective, cross-sectional and analytical study including children aged between 6 and 16-years-old, diagnosed with CKD on automated peritoneal dialysis in the Paediatric Nephrology Department of the UMAE, Hospital General Centro Médico La Raza. Patients did not have oedema, congenital heart disease or known dyslipidaemia. In all cases, the adherence booklet showed a negative total daily fluid balance during at least the 2 weeks before the study, and none of the patients had been hospitalised due to fluid overload or hypertension in the previous month. The parents were asked about the cause of the CKD, the length of time since diagnosis and the time on renal replacement therapy with dialysis. After fasting for at least 12h, the weight and height were measured, their blood pressure was determined and the following lab tests were conducted: haemoglobin, glucose, urea, creatinine, ureic nitrogen, calcium, phosphorus, cholesterol, triglycerides, HDL-cholesterol, LDL-cholesterol, C-reactive protein and parathyroid hormone. The blood levels were determined by the automated colorimetric method using Roche-DiagnosticsTM Modular PP equipment (Japan), by photometry and ion selective electrode (ISE) 900 P800 and by a solid-phase sequential chemiluminescent immunometric assay using IMMUNILITE 2000TM equipment. The left ventricular ejection fraction (LVEF) and shortening fraction (SF), and the E and E′ waves in the mitral valve were measured using Philips iE33TM two-dimensional M-mode ultrasound, with colour-flow, pulsed-wave, continuous-wave and tissue Doppler (Netherlands), with a 3mHz linear transducer, always by the same observer. The E/E′ ratio was considered increased when it was greater than 15. The association between E/E′ and the different variables was evaluated using the Student-t and Mann–Whitney U tests, Pearson's R test and Spearman's Rho test, and the Chi-square and Fisher's exact tests. Multiple linear regression model was used to determine what variables were independent predictors of E/E′. Logistic regression was used to determine what variables were associated with a risk of increased E/E′. The SPSS statistics package (version 20.0) was used, and p values less than 0.05 were considered significant.
ResultsTwenty-nine patients were included in the study, 19 (65.5%) were females, the age was 14±2.6 on renal replacement therapy for 16.89±11.27 months. The average left ventricular ejection fraction was 76.0±7.5, SF 43.10±7.6 and E/E′ ratio of 19.23±7.69. Although only one patient (3.4%) had a reduced left ventricular ejection fraction, 21 (72.4%) patients already had an increased E/E′ ratio.
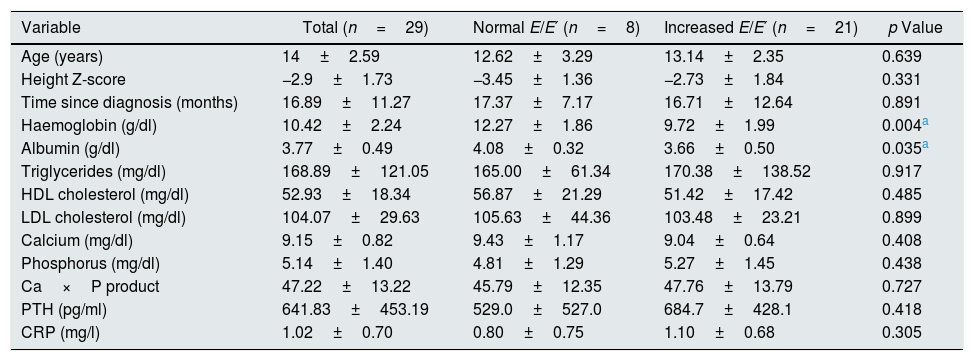
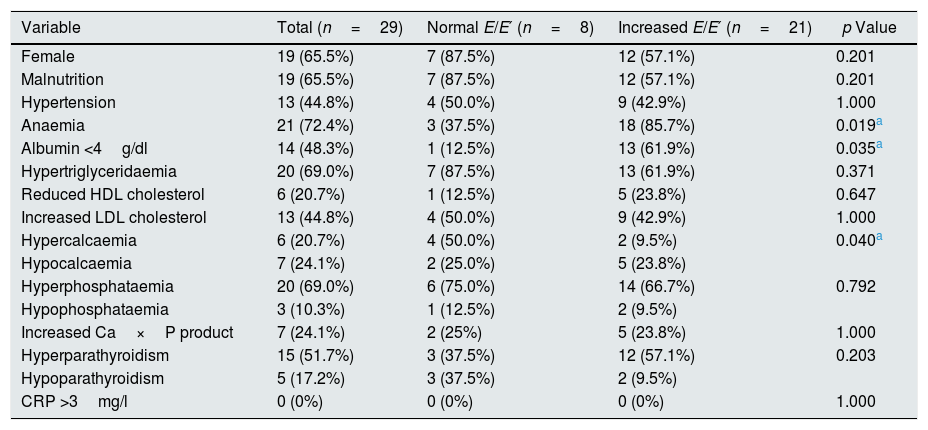
The haemoglobin and albumin values were significantly lower in patients with an increased E/E′ ratio as compared to those who had a normal E/E′ ratio (Table 1). The rate of hypoalbuminaemia and anaemia was higher in patients with an increased E/E′ ratio, while the prevalence of hypercalcaemia was significantly lower in patients with increased E/E′ (Table 2).
Values of the biochemical and echocardiographic variables.
| Variable | Total (n=29) | Normal E/E′ (n=8) | Increased E/E′ (n=21) | p Value |
|---|---|---|---|---|
| Age (years) | 14±2.59 | 12.62±3.29 | 13.14±2.35 | 0.639 |
| Height Z-score | −2.9±1.73 | −3.45±1.36 | −2.73±1.84 | 0.331 |
| Time since diagnosis (months) | 16.89±11.27 | 17.37±7.17 | 16.71±12.64 | 0.891 |
| Haemoglobin (g/dl) | 10.42±2.24 | 12.27±1.86 | 9.72±1.99 | 0.004a |
| Albumin (g/dl) | 3.77±0.49 | 4.08±0.32 | 3.66±0.50 | 0.035a |
| Triglycerides (mg/dl) | 168.89±121.05 | 165.00±61.34 | 170.38±138.52 | 0.917 |
| HDL cholesterol (mg/dl) | 52.93±18.34 | 56.87±21.29 | 51.42±17.42 | 0.485 |
| LDL cholesterol (mg/dl) | 104.07±29.63 | 105.63±44.36 | 103.48±23.21 | 0.899 |
| Calcium (mg/dl) | 9.15±0.82 | 9.43±1.17 | 9.04±0.64 | 0.408 |
| Phosphorus (mg/dl) | 5.14±1.40 | 4.81±1.29 | 5.27±1.45 | 0.438 |
| Ca×P product | 47.22±13.22 | 45.79±12.35 | 47.76±13.79 | 0.727 |
| PTH (pg/ml) | 641.83±453.19 | 529.0±527.0 | 684.7±428.1 | 0.418 |
| CRP (mg/l) | 1.02±0.70 | 0.80±0.75 | 1.10±0.68 | 0.305 |
CRP: C-reactive protein; PTH: parathyroid hormone.
The Student's t-test was used for independent samples.
Prevalence of cardiovascular risk factors.
| Variable | Total (n=29) | Normal E/E′ (n=8) | Increased E/E′ (n=21) | p Value |
|---|---|---|---|---|
| Female | 19 (65.5%) | 7 (87.5%) | 12 (57.1%) | 0.201 |
| Malnutrition | 19 (65.5%) | 7 (87.5%) | 12 (57.1%) | 0.201 |
| Hypertension | 13 (44.8%) | 4 (50.0%) | 9 (42.9%) | 1.000 |
| Anaemia | 21 (72.4%) | 3 (37.5%) | 18 (85.7%) | 0.019a |
| Albumin <4g/dl | 14 (48.3%) | 1 (12.5%) | 13 (61.9%) | 0.035a |
| Hypertriglyceridaemia | 20 (69.0%) | 7 (87.5%) | 13 (61.9%) | 0.371 |
| Reduced HDL cholesterol | 6 (20.7%) | 1 (12.5%) | 5 (23.8%) | 0.647 |
| Increased LDL cholesterol | 13 (44.8%) | 4 (50.0%) | 9 (42.9%) | 1.000 |
| Hypercalcaemia | 6 (20.7%) | 4 (50.0%) | 2 (9.5%) | 0.040a |
| Hypocalcaemia | 7 (24.1%) | 2 (25.0%) | 5 (23.8%) | |
| Hyperphosphataemia | 20 (69.0%) | 6 (75.0%) | 14 (66.7%) | 0.792 |
| Hypophosphataemia | 3 (10.3%) | 1 (12.5%) | 2 (9.5%) | |
| Increased Ca×P product | 7 (24.1%) | 2 (25%) | 5 (23.8%) | 1.000 |
| Hyperparathyroidism | 15 (51.7%) | 3 (37.5%) | 12 (57.1%) | 0.203 |
| Hypoparathyroidism | 5 (17.2%) | 3 (37.5%) | 2 (9.5%) | |
| CRP >3mg/l | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
CRP: C-reactive protein; HDL: high-density lipoproteins; LDL: low-density lipoproteins.
The Chi-square or Fisher's exact test were used.
The correlation analysis revealed statistically significant associations between left ventricular ejection fraction and haemoglobin (r=0.44, p=0.017) and albumin (r=0.58, p=0.001). The SF correlated with haemoglobin (r=0.46, p=0.012) and albumin (r=0.56, p=0.001) and the E/E′ ratio was correlated with haemoglobin (r=–0.53, p=0.003).
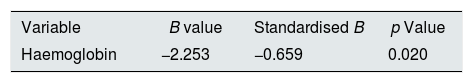
Haemoglobin was the only independent predictor of the E/E′ ratio; for every 1g/dl reduction of Hg the E/E′ ratio increased by 0.66 units (Table 3). Patients who presented with anaemia were 10 times more likely to have an increased E/E′ ratio than patients who did not have anaemia (95% CI 1.52–65.67), with this being the only cardiovascular risk factor showing a higher probability of having an increased E/E′ (Table 4).
Linear regression of the E/E′ with the risk factors in the patients studied.
| Variable | B value | Standardised B | p Value |
|---|---|---|---|
| Haemoglobin | −2.253 | −0.659 | 0.020 |
Variables included in the model: Gender, age, height Z-score, time since CKD diagnosis, haemoglobin, hypertension, albumin, triglycerides, HDL-cholesterol, LDL-cholesterol, Cav×P product, parathyroid hormone and C-reactive protein.
p Values <0.05 were considered significant.
Logistic regression of E/E′ with the risk factors in the patients studied.
| Variable | B value | p Value | OR | 95% CI |
|---|---|---|---|---|
| Anaemia | 2.303 | 0.016 | 10.00 | 1.52–65.67 |
Variables included in the model: gender, age, malnutrition, time since CKD diagnosis, anaemia, hypertension, hypoalbuminaemia, hypertriglyceridaemia, low HDL-cholesterol, high LDL-cholesterol, increased Ca×P product, increased and reduced parathyroid hormone, increased C-reactive protein.
p Values <0.05 were considered significant.
It has been reported that LVDD is more common than ventricular dysfunction with a reduced ejection fraction.3 In this study, we found that while only one patient had a reduced left ventricular ejection fraction, 21 (72.4%) already had an increased E/E′ ratio as a factor of LVDD. This also coincides with the 79% LVDD frequency reported by Jan Ten Harkel et al. in 14 children undergoing peritoneal dialysis.21
With respect to the risk factors investigated, we found a consistent association between anaemia and E/E′. In adults with CKD, there is a negative correlation between anaemia and left ventricular hypertrophy (LVH).10,22The risk of death is also higher in patients who have anaemia and heart failure.22,23 An observational study of patients with severe LVH revealed that children with lower levels of haemoglobin had more severe LVH and a decreased ventricular compliance.4 In children with CKD, an improvement in anaemia is associated to an improvement in cardiac geometry.11 A blind cross-over study of 11 patients aged between 2 and 12 on dialysis revealed an improvement in the heart rate of anaemic children treated with erythropoietin.12 Anaemia causes the heart muscle to work harder due to an increase in heart rate and systolic volume.24 In response, the heart undergoes “remodelling”, which is later reflected in hypertrophy and left ventricular dilatation resulting in heart failure and increased mortality.
Almost 2/3 of our patients were malnourished. Stenvinkel et al. found malnutrition in 18–50% of the groups of patients with CKD undergoing replacement therapy with peritoneal dialysis.13 In our study, the albumin concentrations were significantly correlated with the SF; in patients with increased E/E′ these were considerably lower and the proportion of patients with hypoalbuminaemia was greater. As far as we know, no other study had previously reported a significant association between blood albumin concentrations and LVDD in children undergoing dialysis treatment. Low levels of albumin have been used as markers of malnutrition for many years. Hypoalbuminaemia is present in up to one third of patients with heart failure,25 and it has been consistently associated with an increase in mortality in older adults.26,27 The blood albumin level is an independent predictor of one-year survival in patients with heart failure, even with preserved ejection fraction.28 Blood albumin was found to be closely related with the inflammation in chronic diseases, with hypoalbuminaemia being an indicator of a poor prognosis in heart failure.29 In addition to the consensus that hypoalbuminaemia and inflammation I associated to each other in patients with chronic diseases, and that one condition could even induce the presence of the other, it has been demonstrated that cachexia, evaluated using bioimpedance vector analysis, is independently associated to death even in patients with stable chronic heart failure.30 In a previous study involving children with chronic kidney disease, it was demonstrated that patients with hypoalbuminaemia had a 4-fold higher risk of presenting with greater intima-media thickness of the carotid arteries than those who had normal albumin, both in stages 2–4 of the disease and in stage 5 already undergoing peritoneal dialysis or haemodialysis, all adjusted for possible confounders, including blood levels of interleukin 1β and 6 and high-sensitivity C-reactive protein.15 Growing evidence suggests that severe hypoalbuminaemia has pleiotropic effects on many organs and neurohumoral systems involved in heart failure syndrome. Capillary hydrostatic pressure and plasma oncotic pressure are the main opposing forces that regulate the fluid balance throughout the capillary membrane, and there is definitive evidence that severe hypoalbuminaemia leads to pulmonary oedema. Serum albumin helps to maintain the integrity of the microvasculature of the myocardium through its oncotic properties and its interaction with the endothelial glycocalyx.29 Severe hypoalbuminaemia aggravates the myocardial oedema, which is considered one of the causes of myocardial dysfunction and electrophysiological instability in many heart diseases.29,31 It can exacerbate oxidative stress and inflammation that are involved in the overall heart failure process,29,32,33 and contributes to fluid overload by activating baroreceptors and resistance to diuretics.29
Given the socio-economic characteristics of our patients, we can not underestimate the role of malnutrition as a cause of anaemia, hypoalbuminaemia and LVDD. In order to prevent the onset of LVDD due to anaemia, it is essential to ensure adequate iron intake from food and supplements, sufficient bodily reserves, adequate control of blood pressure, achieved through the continuous administration of erythropoietin, as well as exhaustive investigation and treatment of other specific factors of the disease that may cause imperceptible losses, affect the reserves and hinder the utilisation of iron. It is essential, from the very early stages of chronic kidney disease, to promote the preservation and improvement of the nutritional status, by providing food and supplements in the form and by the means necessary to ensure adequate nutrition. Furthermore, permanent prevention, active investigation, early diagnosis and proper treatment of any source of inflammation are vital.
We concluded that the LVDD was present in 3/4 of children with CKD treated with automated peritoneal dialysis, even when the ejection fraction frequency was low. Anaemia and hypoalbuminaemia are significantly linked to LVDD. It is necessary to conduct prospective and longitudinal studies including large number of children at various stages of chronic kidney disease on nutritional supplements to analyse to what extent the change in nutritional status and in the clinical and biochemical markers affects left ventricular diastolic function.
Conflicts of interestThe authors state that they have no conflicts of interest with regard to this study.
Please cite this article as: García-Bello JA, Ortiz-Flores J, Torres de la Riva FE, Mendoza-Moreno GK, Gómez-Tenorio C. Anemia e hipoalbuminemia como factores de riesgo de disfunción diastólica del ventrículo izquierdo en niños con insuficiencia renal crónica tratados con diálisis peritoneal. Nefrologia. 2018;38:408–413.