Chronic kidney disease (CKD) is a public health problem worldwide. We aimed to estimate the CKD prevalence in Spain and to examine the impact of the accumulation of cardiovascular risk factors (CVRF).
Material and methodsWe performed a nationwide, population-based survey evaluating 11,505 individuals representative of the Spanish adult population. Information was collected through standardized questionnaires, physical examination, and analysis of blood and urine samples in a central laboratory. CKD was graded according to current KDIGO definitions. The relationship between CKD and 10CVRF was assessed (age, hypertension, general obesity, abdominal obesity, smoking, high LDL-cholesterol, low HDL-cholesterol, hypertriglyceridemia, diabetes and sedentary lifestyle).
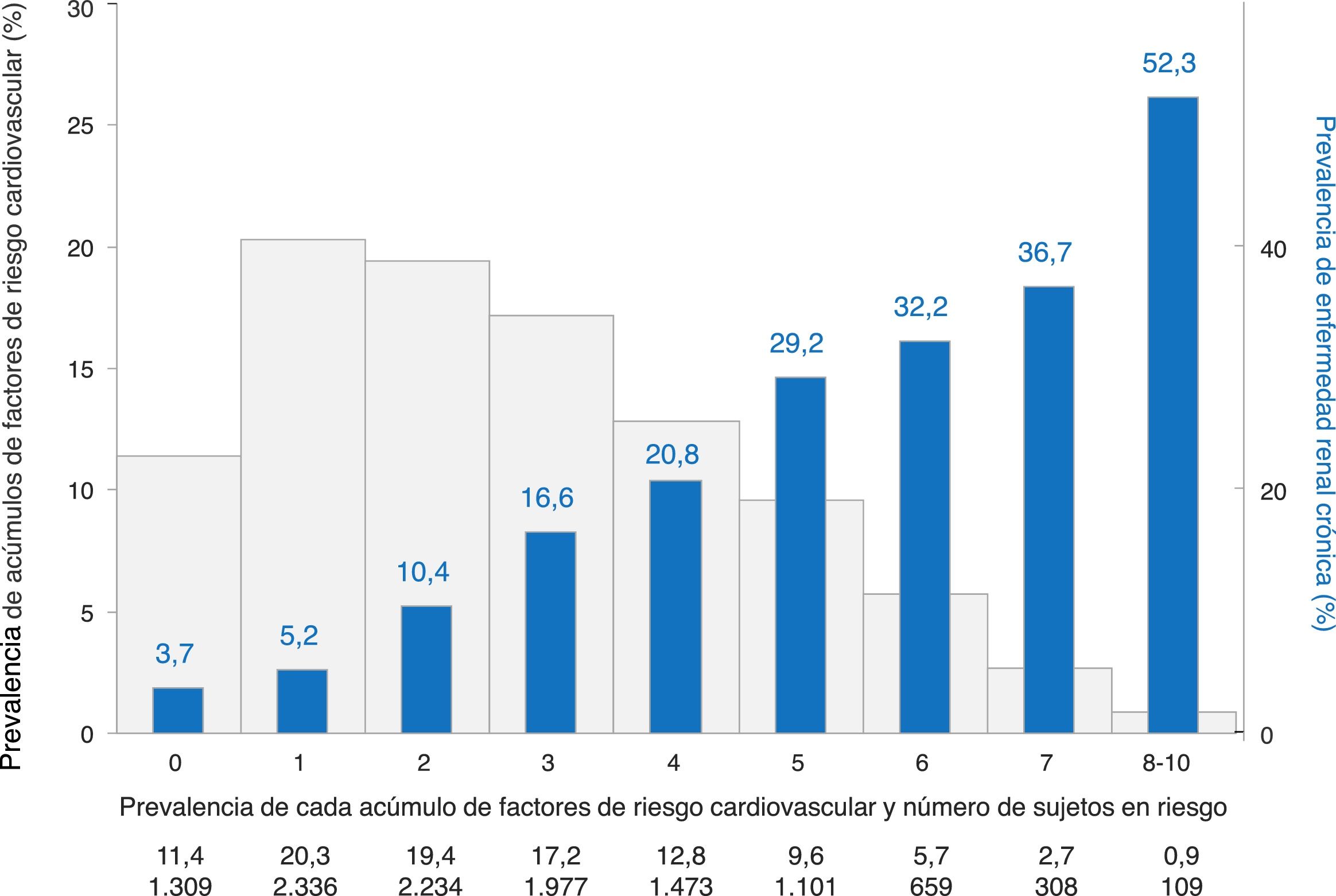
ResultsPrevalence of CKD was 15.1% (95% CI: 14.3–16.0%). CKD was more common in men (23.1% vs. 7.3% in women), increased with age (4.8% in 18–44 age group, 17.4% in 45–64 age group, and 37.3% in ≥65), and was more common in those with than those without cardiovascular disease (39.8% vs. 14.6%); all P<0.001. CKD affected 4.5% of subjects with 0–1CVRF, and then progressively increased from 10.4% to 52.3% in subjects with 2 to 8–10CVRF (P trend <0.001).
ConclusionsCKD affects one in seven adults in Spain. The prevalence is higher than previously reported and similar to that in the United States. CKD was particularly prevalent in men, older people and people with cardiovascular disease. Prevalence of CKD increased considerably with the accumulation of CVRF, suggesting that CKD could be considered as a cardiovascular condition.
La enfermedad renal crónica (ERC) constituye un problema de salud pública a nivel mundial. Los objetivos de este estudio fueron estimar la prevalencia de ERC en España y evaluar el impacto de la acumulación de factores de riesgo cardiovascular (FRCV) en la prevalencia.
Material y métodosAnálisis del Estudio de Nutrición y Riesgo Cardiovascular en España (ENRICA), estudio epidemiológico de ámbito nacional, de base poblacional, con una muestra de 11.505 sujetos representativos de la población adulta española. La información se recogió mediante cuestionarios estandarizados, exploración física y colección de muestras de sangre y orina que se analizaron en un laboratorio centralizado. La ERC se definió según las guías KDIGO en curso. Se analizó la relación de la ERC con 10FRCV (edad, hipertensión arterial, obesidad, obesidad abdominal, tabaquismo, colesterol LDL elevado, colesterol HDL disminuido, hipertrigliceridemia, diabetes y sedentarismo.
ResultadosLa prevalencia de ERC fue del 15,1% (IC95%: 14,3-16,0). La ERC fue más frecuente en varones (23,1% vs. 7,3% en mujeres), según aumentaba la edad (4,8% en sujetos de 18-44años, 17,4% en sujetos de 45-64años, y 37,3% en sujetos ≥65años), y en sujetos con enfermedad cardiovascular (39,8% vs. 14,6% en sujetos sin enfermedad cardiovascular); todas las comparaciones con p<0,001. La ERC afectó al 4,5% de los sujetos con 0-1FRCV, con un aumento progresivo desde el 10,4 al 52,3% en sujetos con 2 a 8-10FRCV (p de tendencia <0,001).
ConclusionesLa ERC afecta a uno de cada 7 adultos en España, una prevalencia más elevada que la estimada en estudios previos en nuestro país y similar a la observada en Estados Unidos. La ERC afecta particularmente a los varones, a sujetos de edad avanzada o con enfermedad cardiovascular. La prevalencia de ERC aumenta de forma marcada con la acumulación de FRCV, lo que sugiere que la ERC en la población podría considerarse como un trastorno cardiovascular.
In the past two decades, chronic kidney disease (CKD) has become a leading public health problem worldwide.1,2 The burden of CKD basically results from two components. First, treatment of advanced CKD, also termed as chronic kidney failure or end-stage renal disease (ESRD), which requires dialysis and/or kidney transplantation; it affects to only 1% of subjects with CKD, but dramatically reduces life expectancy and constitutes one of the most expensive treatment for chronic disease. In fact, this account for 5% of the annual health budget, which is spent on less than 1% of the population.1 Second, CKD, even in early stages, is closely associated with higher total and cardiovascular mortality and morbidity in the general population and in subjects with increased risk of cardiovascular disease (CVD); thus it affects to a large number of individuals.2,3
Early identification of CKD is considered a priority in public health to slow progression to ESRD and to prevent CVD morbidity and mortality. Reduction of burden and costs of CKD should be included in national health programs,4 and estimation of CKD prevalence in the population is of key importance to support prevention and management of CKD in healthcare systems.5,6
An analysis of worldwide data has shown that nearly 500 million adults suffer from CKD.7 Several studies have reported a wide variation in the prevalence of CKD across European and non-European countries,8–10 which might be due to clinical, environmental, geographic, and even socio-economic variables, but also to methodological issues.4,7,10 A recent report of the European CKD Burden Consortium recommended methodological standards to harmonize reporting of CKD prevalence.6 Furthermore, CKD is usually associated with major CVD risk factors,11–13 but few studies have delved into this aspect that is promising for prevention.
Previous European studies do not reflect the actual prevalence of CKD in European countries such as Spain.10 Thus, we used a population-based, nationally representative survey of the adult population of Spain (i) to estimate the prevalence of CKD according to currents definition of CKD and reporting methodology,1,6 and (ii) to examine the impact of the increasing number of CVD risk factors on the prevalence of CKD. All these aspects could have important clinical and public health implications for planning care and prevention of CKD at the population level.
MethodsStudy design and participantsData were taken from the ENRICA (Study on Nutrition and Cardiovascular Risk in Spain), whose methods have been reported previously.14–17 Briefly, this is a cross-sectional study conducted from June 2008 to October 2010 with 12,948 individuals representative of the non-institutionalized population of Spain aged ≥18 years. Participants were selected by multistage clustered random sampling. The sample was first stratified by province and by size of municipality. Clusters were then selected randomly in 2 stages, municipalities and census sections. Finally, the households within each section were selected by random landline telephone dialing. Subjects in the households were selected proportionally to sex- and age-distribution of the population of Spain. Information was collected by telephone interview, face-to-face interview, physical examination, and by taking blood and urine samples in the households.
The study protocol was approved by the clinical research ethics committees of the Hospital Universitario La Paz in Madrid and the Hospital Clínic in Barcelona. All study participants gave written informed consent for the study.
Data collection and variable definitionWeight, height, and waist circumference were measured twice in each subject under standardized conditions using electronic scales (model Seca 841, precision to 0.1kg), portable extendable stadiometers (model Ka We 44 444Seca), and flexible, inelastic belt-type tapes. Body mass index (BMI) was calculated as weight in kg divided by squared height in m.
Blood pressure (BP) measurement was performed by certified trained personnel, using standardized procedures with validated automatic devices (model Omron M6) and cuffs of three sizes according to arm circumference. Two sets of BP readings were made separated by 90min. In each set, BP was measured 3 times at 1–2min intervals, after a 3–5min resting period. For analyses, BP was calculated as the mean of the last five readings. Study participants reported their smoking status. Physical activity was assessed with the questionnaire from the EPIC (European Prospective Investigation into Cancer) study in Spain,18 which combines physical activity at work and during leisure time into 4 levels (very active, moderately active, moderately inactive, and inactive). Individuals also reported physician-diagnosed CVD. All variables were defined according to international guidelines.19,20
Laboratory and kidney function measurementsParticipants provided 12-hour fasting blood and spot urine samples at their homes. Laboratory determinations were performed centrally at the Center of Biological Diagnosis of the Hospital Clínic in Barcelona, using standard procedures and appropriate quality controls.14–17 Serum glucose, hemoglobin A1c, total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were determined by enzymatic methods, and low-density lipoprotein (LDL) cholesterol by the Friedewald formula. Creatinine was determined by Jaffé, alcalin picrate by kinetic reaction, and microalbumin by polyethylene glycol-enhanced immunoturbidimetry.
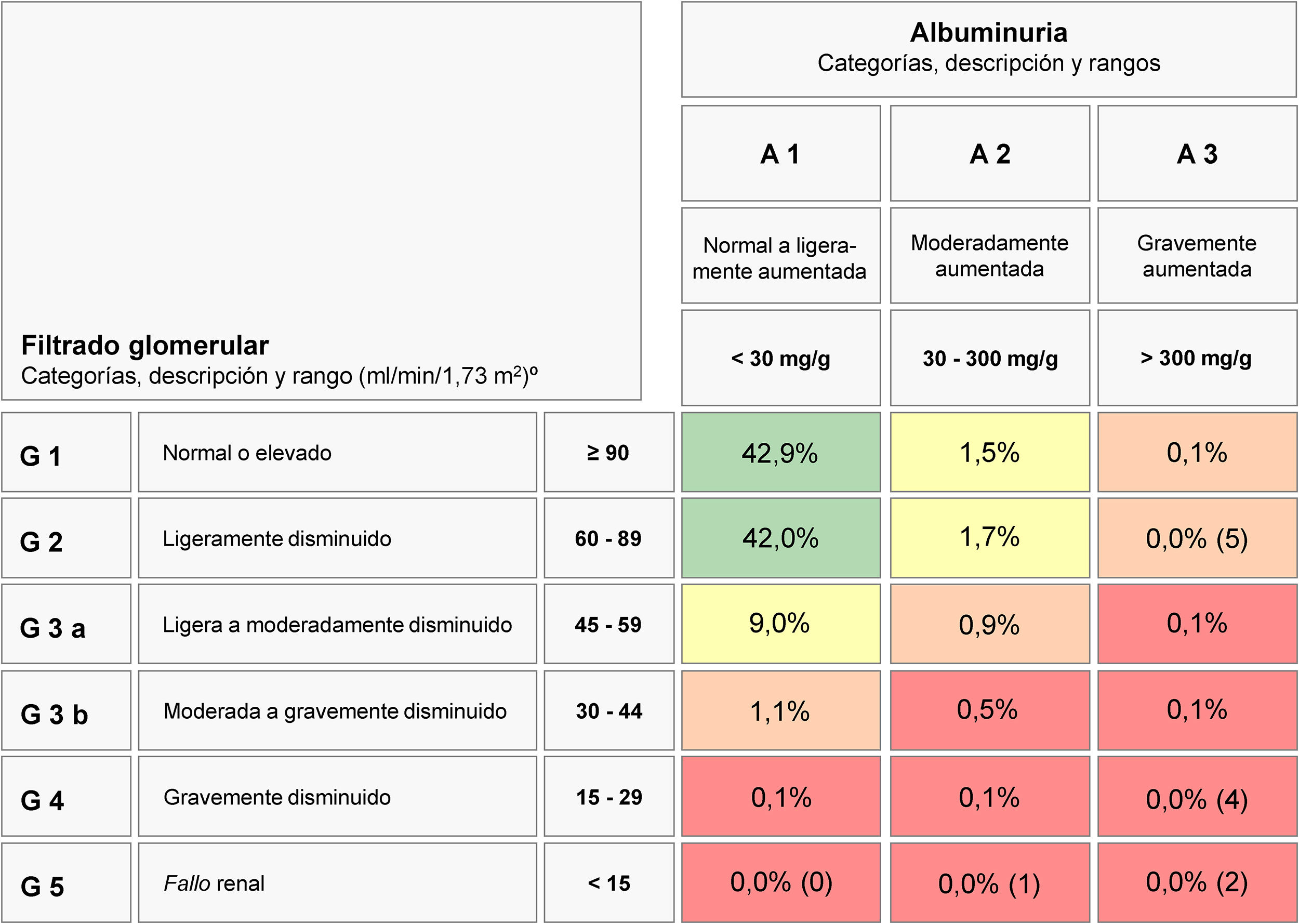
CKD was defined according to current Kidney Disease: Improving Global Outcomes (KDIGO) guidelines using estimated glomerular filtration rate (eGFR) calculated with the CKD – Epidemiology Collaboration (CKD-EPI) equation, and albuminuria measured by the albumin-to-creatinine ratio (ACR).1 CKD was defined as either a low eGFR (<60mL/min/1.73m2) or an increased ACR (≥30mg/g). Subjects were classified according the KDIGO GFR 6-category staging (grades G1, G2, G3a, G3b, G4, and G5 corresponding to eGFR ≥90, 60–89, 45–59, 30–44, 15–29, and <15mL/min/1.73m2, respectively), and albuminuria 3-category staging (A1, A2, and A3 corresponding to ACR <30, 30–300, and >300mg/g, respectively). Then, 6 CKD grades were established (G1 CKD for G1 eGFR with increased albuminuria A2 or A3, G2 CKD for G2 eGFR with increased albuminuria A2 or A3, G3a CKD for G3a eGFR with either normoalbuminuria A1 or increased albuminuria A2 or A3, G3b CKD for G3b eGFR with either normoalbuminuria A1 or increased albuminuria A2 or A3, G4 CKD for G4 eGFR with either normoalbuminuria A1 or increased albuminuria A2 or A3, and G5 CKD for G5 eGFR with either normoalbuminuria A1 or increased albuminuria A2 or A3. Subjects were also classified according the prognostic KDIGO 6 × 3 table (6 eGFR categories × 3 albuminuria categories) to estimate percentages of subjects at moderately increased risk, high risk, and very high risk of complications from CKD.
Cardiovascular risk definitionsA total of 10 classic CVD risk factors were assessed: age, hypertension, general obesity, abdominal obesity, current smoking, diabetes, increased LDL cholesterol, low HDL-cholesterol, hypertriglyceridemia, and sedentary habit. Risk age was considered >65 years for women and >55 years for men. Hypertension was defined as systolic BP ≥140mmHg, and/or diastolic BP ≥90mmHg, and/or on current antihypertensive drug treatment.19 Obesity was defined as BMI was ≥30kg/m2, and abdominal obesity as waist circumference was >88cm in women and >102cm in men. Diabetes was defined as glucose ≥126mg/dL or glycosylated hemoglobin ≥6.5%, or using oral antidiabetic drugs or insulin.20 Increased LDL cholesterol was >115mg/dL, low HDL-cholesterol was <46mg/dL in women and <40mg/dL in men, and hypertriglyceridemia was >150mg/dL, and hypercholesterolemia was total cholesterol >190mg/dL, or receiving lipid-lowering drugs.19 Sedentary habit was considered when physical activity was less than moderate. In participants with no previous CVD, the 10-year risk of CVD death was estimated using the SCORE (Systematic COronary Risk Evaluation) equation for low-risk European regions, which includes Spain, being high risk considered when risk was ≥5%.19
Statistical analysesOf the 12,948 study participants, the following were excluded because lacking data: 1251 on BP, sociodemographic or clinical variables, or laboratory values other than kidney-related parameters, and 192 on serum creatinine or albuminuria. Thus, 11,505 individuals were valid for the analyses (88.9% of the total sample).
Analyses accounted for the complex sampling design, so individual observations were weighted to reconstruct the Spanish population, and the variances were corrected to obtain appropriate 95% confidence intervals (CI) for the main results. The Student t test and ANOVA were used to compare means and the χ2 test was used to compare proportions. Trends in prevalence of CKD across stage, age, and number of CV risk factors were also calculated (P for trend).
Finally, we performed multivariate logistic regression of factors associated with CKD. Sociodemographic variables and cardiovascular risk factors that were statistically significant in univariate analyses or considered a priori clinically relevant were selected for multivariable analyses. Results were summarized with odds ratios and their 95% confidence intervals [CI].
Statistical significance was set at 2-sided P<0.05. The analyses were performed with the SPSS package 18.0 (IBM, Armonk, NY).
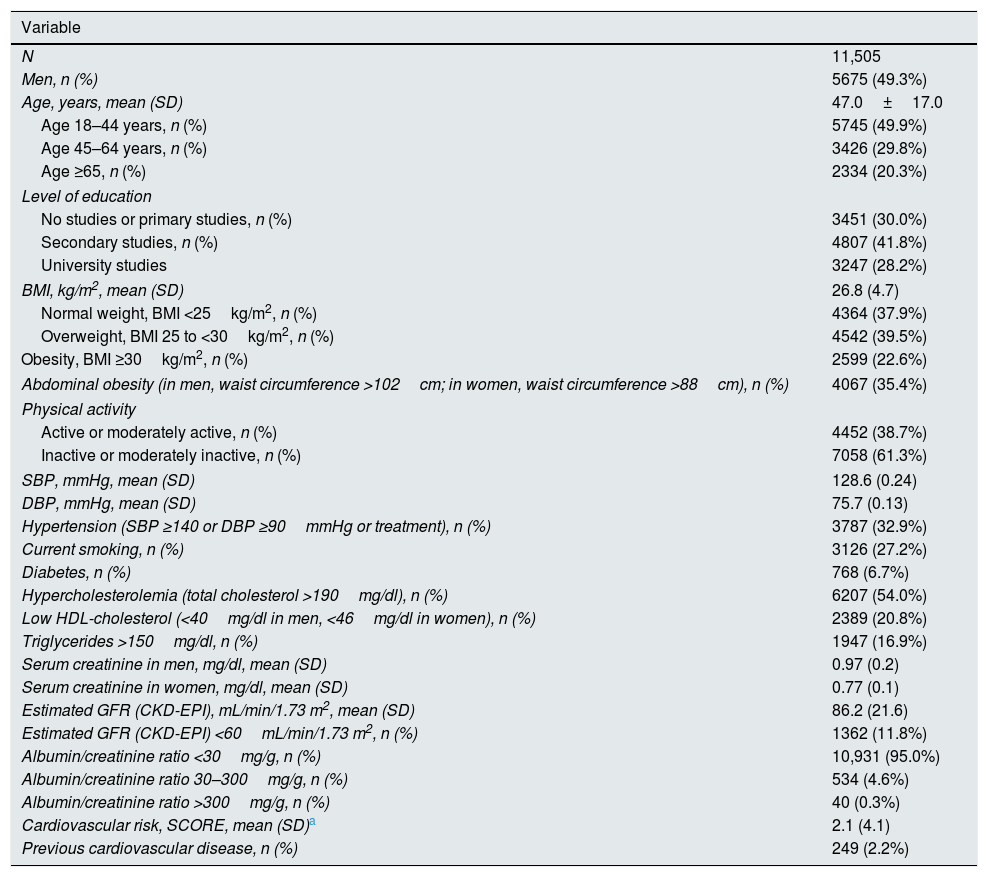
ResultsTable 1 shows main characteristics of study participants. They had a mean age of 47 years, 20.3% were older than 65 years, and 50.7% were women. About 23% of individuals had obesity and 35.4% abdominal obesity. Moderated to severe sedentary habit affected more than 60% of study participants. One out of three individuals showed hypertension, being the prevalence of diabetes, smoking habit, and hypercholesterolemia 6.7%, 27.2%, and 54.0%, respectively. CVD was present in 2.2% of subjects.
Characteristics of the study participants.
| Variable | |
|---|---|
| N | 11,505 |
| Men, n (%) | 5675 (49.3%) |
| Age, years, mean (SD) | 47.0±17.0 |
| Age 18–44 years, n (%) | 5745 (49.9%) |
| Age 45–64 years, n (%) | 3426 (29.8%) |
| Age ≥65, n (%) | 2334 (20.3%) |
| Level of education | |
| No studies or primary studies, n (%) | 3451 (30.0%) |
| Secondary studies, n (%) | 4807 (41.8%) |
| University studies | 3247 (28.2%) |
| BMI, kg/m2, mean (SD) | 26.8 (4.7) |
| Normal weight, BMI <25kg/m2, n (%) | 4364 (37.9%) |
| Overweight, BMI 25 to <30kg/m2, n (%) | 4542 (39.5%) |
| Obesity, BMI ≥30kg/m2, n (%) | 2599 (22.6%) |
| Abdominal obesity (in men, waist circumference >102cm; in women, waist circumference >88cm), n (%) | 4067 (35.4%) |
| Physical activity | |
| Active or moderately active, n (%) | 4452 (38.7%) |
| Inactive or moderately inactive, n (%) | 7058 (61.3%) |
| SBP, mmHg, mean (SD) | 128.6 (0.24) |
| DBP, mmHg, mean (SD) | 75.7 (0.13) |
| Hypertension (SBP ≥140 or DBP ≥90mmHg or treatment), n (%) | 3787 (32.9%) |
| Current smoking, n (%) | 3126 (27.2%) |
| Diabetes, n (%) | 768 (6.7%) |
| Hypercholesterolemia (total cholesterol >190mg/dl), n (%) | 6207 (54.0%) |
| Low HDL-cholesterol (<40mg/dl in men, <46mg/dl in women), n (%) | 2389 (20.8%) |
| Triglycerides >150mg/dl, n (%) | 1947 (16.9%) |
| Serum creatinine in men, mg/dl, mean (SD) | 0.97 (0.2) |
| Serum creatinine in women, mg/dl, mean (SD) | 0.77 (0.1) |
| Estimated GFR (CKD-EPI), mL/min/1.73 m2, mean (SD) | 86.2 (21.6) |
| Estimated GFR (CKD-EPI) <60mL/min/1.73 m2, n (%) | 1362 (11.8%) |
| Albumin/creatinine ratio <30mg/g, n (%) | 10,931 (95.0%) |
| Albumin/creatinine ratio 30–300mg/g, n (%) | 534 (4.6%) |
| Albumin/creatinine ratio >300mg/g, n (%) | 40 (0.3%) |
| Cardiovascular risk, SCORE, mean (SD)a | 2.1 (4.1) |
| Previous cardiovascular disease, n (%) | 249 (2.2%) |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GFR, glomerular filtration rate; CKD-EPI, Chronic Kidney Disease – Epidemiology Collaboration.
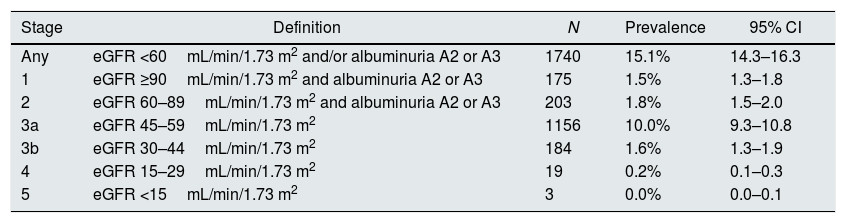
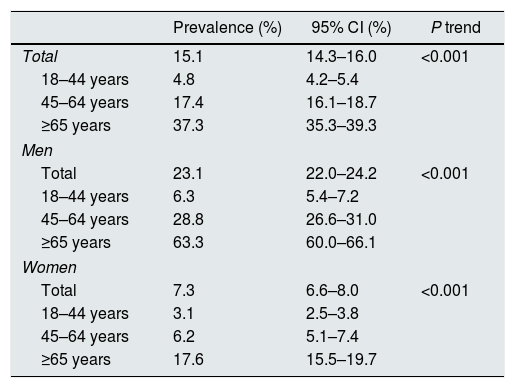
CKD was present in 15.1% of individuals (95% confidence interval 14.3–16.3%) (Table 2). Stage 3a was the more frequent grade of CKD affecting 10.0% of subjects. Prevalences of stages 1, 2, 3b, 4, and 5 were 1.5%, 1.8%, 1.6%, 0.2%, and 0.0%, respectively. Prevalence of CKD was more than 3-fold higher in men than in women (23.1% vs. 7.3%), and sharply increased with age (Table 3). In every age strata, CKD was more frequent in men than in women, being these differences particularly wider in the middle age group (4.6 times higher), and in the older age group (3.6 times higher). Also, CKD prevalence was more frequent among those with than without CVD (39.8% vs. 14.6%, respectively, P<0.001).
Prevalence of chronic kidney disease.
| Stage | Definition | N | Prevalence | 95% CI |
|---|---|---|---|---|
| Any | eGFR <60mL/min/1.73 m2 and/or albuminuria A2 or A3 | 1740 | 15.1% | 14.3–16.3 |
| 1 | eGFR ≥90mL/min/1.73 m2 and albuminuria A2 or A3 | 175 | 1.5% | 1.3–1.8 |
| 2 | eGFR 60–89mL/min/1.73 m2 and albuminuria A2 or A3 | 203 | 1.8% | 1.5–2.0 |
| 3a | eGFR 45–59mL/min/1.73 m2 | 1156 | 10.0% | 9.3–10.8 |
| 3b | eGFR 30–44mL/min/1.73 m2 | 184 | 1.6% | 1.3–1.9 |
| 4 | eGFR 15–29mL/min/1.73 m2 | 19 | 0.2% | 0.1–0.3 |
| 5 | eGFR <15mL/min/1.73 m2 | 3 | 0.0% | 0.0–0.1 |
Abbreviations: eGFR, estimated glomerular filtration rate; CI, confidence interval.
A2 indicates a moderately increased urinary albumin excretion rate (albumin/creatinine ratio 30–300mg/g); A3 indicates a severely increased urinary albumin excretion rate (albumin/creatinine ratio >300mg/g).
Prevalence of chronic kidney disease by sex and group of age.
| Prevalence (%) | 95% CI (%) | P trend | |
|---|---|---|---|
| Total | 15.1 | 14.3–16.0 | <0.001 |
| 18–44 years | 4.8 | 4.2–5.4 | |
| 45–64 years | 17.4 | 16.1–18.7 | |
| ≥65 years | 37.3 | 35.3–39.3 | |
| Men | |||
| Total | 23.1 | 22.0–24.2 | <0.001 |
| 18–44 years | 6.3 | 5.4–7.2 | |
| 45–64 years | 28.8 | 26.6–31.0 | |
| ≥65 years | 63.3 | 60.0–66.1 | |
| Women | |||
| Total | 7.3 | 6.6–8.0 | <0.001 |
| 18–44 years | 3.1 | 2.5–3.8 | |
| 45–64 years | 6.2 | 5.1–7.4 | |
| ≥65 years | 17.6 | 15.5–19.7 | |
Abbreviation: CI, confidence interval.
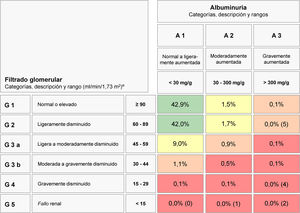
Fig. 1 shows the distribution of CKD by GFR and albuminuria categories of the KDIGO guidelines.1 The majority of subjects with CKD (80.6% of those with CKD, and 12.2% of the total sample) corresponded to cases at moderate risk of CKD complications. About 2.1% of subjects (13.9% of those with CKD) had high risk of CKD complications, and 0.9% (5.5% of those were at very high risk).
Prevalence and number of subjects at different levels of cardio-renal risk by estimated glomerular filtration rate and albuminuria according the CKD 2012 KDIGO guidelines risk stratification score.
eGFR in mL/min/1.73m2; albuminuria in mg/g (albumin/creatinine ratio).
Abbreviations: CKD, chronic kidney disease; KDIGO, Kidney Disease Improving Global Outcomes; eGFR, estimated glomerular filtration rate.
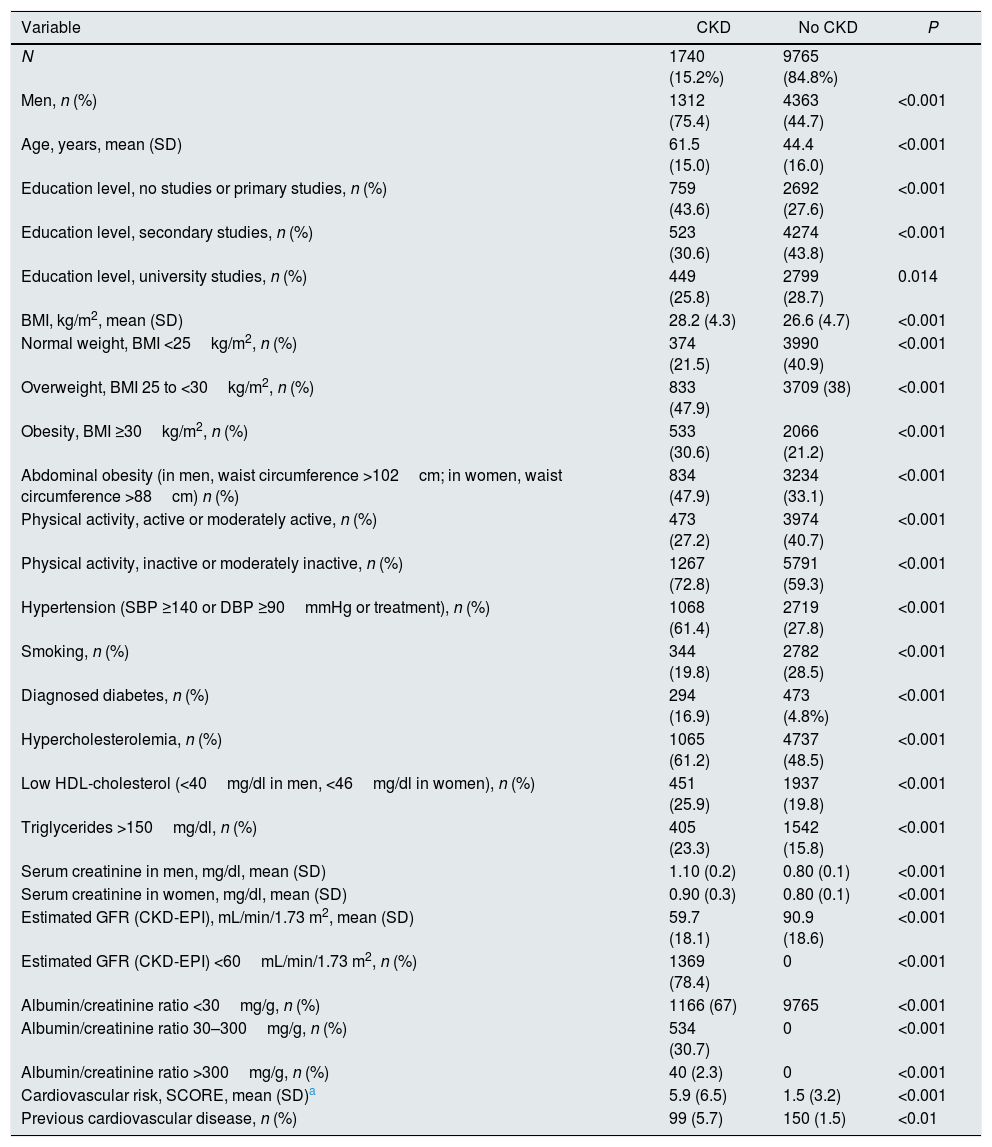
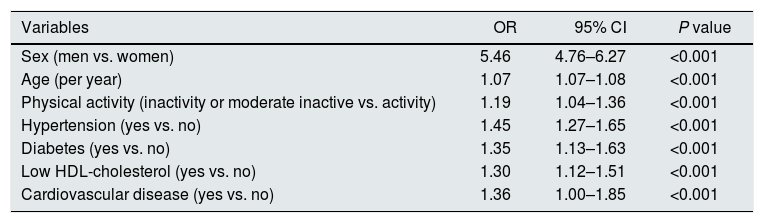
Individuals with CKD were older than subjects with normal renal parameters (61.5 vs. 44.4 years), and were predominantly men (75.4%) (Table 4). Every clinical and analytical variable related to cardiovascular risk was more prevalent within CKD subjects, in particular obesity, hypertension, and diabetes. The exception was smoking, being more frequent within subjects with no CKD, probably due to reverse causality. As a result, cardiovascular risk SCORE was 3 times higher in subjects with CKD. Prevalence of established CVD was nearly 4 times higher in individuals with CKD. Table 5 shows that in multivariable analysis, age, male gender, physically inactivity, hypertension, diabetes, low HDL-cholesterol and CVD, were independently associated with CKD.
Comparison between subjects with and without kidney disease.
| Variable | CKD | No CKD | P |
|---|---|---|---|
| N | 1740 (15.2%) | 9765 (84.8%) | |
| Men, n (%) | 1312 (75.4) | 4363 (44.7) | <0.001 |
| Age, years, mean (SD) | 61.5 (15.0) | 44.4 (16.0) | <0.001 |
| Education level, no studies or primary studies, n (%) | 759 (43.6) | 2692 (27.6) | <0.001 |
| Education level, secondary studies, n (%) | 523 (30.6) | 4274 (43.8) | <0.001 |
| Education level, university studies, n (%) | 449 (25.8) | 2799 (28.7) | 0.014 |
| BMI, kg/m2, mean (SD) | 28.2 (4.3) | 26.6 (4.7) | <0.001 |
| Normal weight, BMI <25kg/m2, n (%) | 374 (21.5) | 3990 (40.9) | <0.001 |
| Overweight, BMI 25 to <30kg/m2, n (%) | 833 (47.9) | 3709 (38) | <0.001 |
| Obesity, BMI ≥30kg/m2, n (%) | 533 (30.6) | 2066 (21.2) | <0.001 |
| Abdominal obesity (in men, waist circumference >102cm; in women, waist circumference >88cm) n (%) | 834 (47.9) | 3234 (33.1) | <0.001 |
| Physical activity, active or moderately active, n (%) | 473 (27.2) | 3974 (40.7) | <0.001 |
| Physical activity, inactive or moderately inactive, n (%) | 1267 (72.8) | 5791 (59.3) | <0.001 |
| Hypertension (SBP ≥140 or DBP ≥90mmHg or treatment), n (%) | 1068 (61.4) | 2719 (27.8) | <0.001 |
| Smoking, n (%) | 344 (19.8) | 2782 (28.5) | <0.001 |
| Diagnosed diabetes, n (%) | 294 (16.9) | 473 (4.8%) | <0.001 |
| Hypercholesterolemia, n (%) | 1065 (61.2) | 4737 (48.5) | <0.001 |
| Low HDL-cholesterol (<40mg/dl in men, <46mg/dl in women), n (%) | 451 (25.9) | 1937 (19.8) | <0.001 |
| Triglycerides >150mg/dl, n (%) | 405 (23.3) | 1542 (15.8) | <0.001 |
| Serum creatinine in men, mg/dl, mean (SD) | 1.10 (0.2) | 0.80 (0.1) | <0.001 |
| Serum creatinine in women, mg/dl, mean (SD) | 0.90 (0.3) | 0.80 (0.1) | <0.001 |
| Estimated GFR (CKD-EPI), mL/min/1.73 m2, mean (SD) | 59.7 (18.1) | 90.9 (18.6) | <0.001 |
| Estimated GFR (CKD-EPI) <60mL/min/1.73 m2, n (%) | 1369 (78.4) | 0 | <0.001 |
| Albumin/creatinine ratio <30mg/g, n (%) | 1166 (67) | 9765 | <0.001 |
| Albumin/creatinine ratio 30–300mg/g, n (%) | 534 (30.7) | 0 | <0.001 |
| Albumin/creatinine ratio >300mg/g, n (%) | 40 (2.3) | 0 | <0.001 |
| Cardiovascular risk, SCORE, mean (SD)a | 5.9 (6.5) | 1.5 (3.2) | <0.001 |
| Previous cardiovascular disease, n (%) | 99 (5.7) | 150 (1.5) | <0.01 |
Abbreviations: CKD, chronic kidney disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GFR, glomerular filtration rate; CKD-EPI, Chronic Kidney Disease – Epidemiology Collaboration.
Multivariate logistic regression analysis assessing correlates of chronic kidney disease.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Sex (men vs. women) | 5.46 | 4.76–6.27 | <0.001 |
| Age (per year) | 1.07 | 1.07–1.08 | <0.001 |
| Physical activity (inactivity or moderate inactive vs. activity) | 1.19 | 1.04–1.36 | <0.001 |
| Hypertension (yes vs. no) | 1.45 | 1.27–1.65 | <0.001 |
| Diabetes (yes vs. no) | 1.35 | 1.13–1.63 | <0.001 |
| Low HDL-cholesterol (yes vs. no) | 1.30 | 1.12–1.51 | <0.001 |
| Cardiovascular disease (yes vs. no) | 1.36 | 1.00–1.85 | <0.001 |
Abbreviations: CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval.
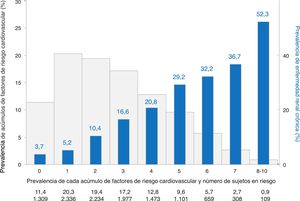
The impact of clustering of up to 10 CVD risk factors on the prevalence of CKD in subjects without CVD is shown in Fig. 2. CKD affected 4.5% of subjects with 0–1 CV risk factors, and then increased from 10.4% to 52.3% in those with 2 to 8–10 CV risk factors (P trend <0.001). The prevalence of CKD in subjects with 3 CVD risk factors (16.6%) was similar to that of the total sample (15.1%). Up to one of three subjects had 4 or more CV risk factors; in this subgroup of individuals prevalence of CKD raised from 21% to more than 50%.
Prevalence of chronic kidney disease according to clustering of cardiovascular risk factors in subjects with no established cardiovascular disease.
Cardiovascular risk factors considered were: age, hypertension, obesity, abdominal obesity, smoking, high LDL-cholesterol, low HDL-cholesterol, high triglycerides, diabetes, and sedentary habit.
This nationwide study, using recent recommendations about methodology for reporting CKD prevalence,6 showed that CKD affected one in seven adults in Spain. Stage 3a CKD was the main responsible of CKD in the population. Prevalence of CKD sharply increased with age, being younger women the subgroup with the lowest prevalence, and older men the subgroup with the highest prevalence. Importantly, the prevalence of CKD increased continuously with the number of CVD risk factors. This is additional evidence consistent with the concept that CKD could be considered as a cardiovascular condition in the population.
Comparison of CKD prevalence with other studiesThe prevalence of CKD worldwide is 11.8% in women and 0.4% in men.7 There are huge geographic differences (from 4.1% in Saudi-Arabian women to 25.7% in men from El Salvador), and by income status (8.6% in men and 9.6% in women in high-income countries vs. 10.6% in men and 12.5% in women in low- and middle-income countries). In China the estimated CKD prevalence in adults was 10.8%.8
In Europe CKD prevalence ranges from 3.3% in Norway to 17.3% in northeast Germany.10 In Spain, the prevalence of CKD in the present study was higher than in a previous and much smaller study conducted in 42 municipalities (9.2%),21 probably reflecting changes over time and methodological differences between studies. In the United States, stage 1–4 CKD prevalence in adults >20 years was 13.1% in the early 2000s, and 13.7% in US Hispanics/Latinos.5,9 From the data available, we can derive that, methodological differences aside, the prevalence of CKD in Spain is comparable to that in the United States and higher than in many European countries.10
Impact of cardiovascular risk factor clustering on CKD prevalence: clinical and population implicationsCVD is common in patients with CKD, and frequently underdiagnosed and undertreated.20 Current European guidelines on CVD prevention22 indicate that ‘microalbuminuria’ and/or a moderately reduced eGFR (30–60mL/min/1.73m2) confers high risk for CVD, and proteinuria and/or a severely reduced eGFR (<30mL/min/1.73m2) should to be considered as very high risk situations. CKD is associated with an increased risk of CVD, independent of conventional CVD risk factors, which are known to be more common in patients with CKD. In addition, inflammatory mediators and promoters of calcification induce vascular damage and may explain the association CKD–CVD even after adjustment for conventional risk factors.13,23
In agreement with previous population-based studies,5,8,9 prevalence of situations at high or very high risk according to KDIGO score where low. In our study the prevalence of an eGFR <30mL/min/1.73m2 was 0.20%, and that of proteinuria was 0.30%. Thus, most of adults showing CKD had situations at moderate risk of cardio-renal complications.
The prevalence of CKD was higher as the number of CVD risk factors increased. Most subjects had 2–6 CVD risk factors and a relatively moderate risk of having CKD. That's where lies the potential for prevention of CKD and its complications using both high-risk individual and moderate-risk population strategies. In particular, as in some other studies,5,8,9,24 people who are older, men, with hypertension, diabetes, low HDL-cholesterol, sedentary, or with CVD are at high risk and should be screened for CKD.
Strengths and limitationsMain strengths of this study are the representativeness of adults at the countrywide level, large sample size, and the use of international methodological standards. This study has also some limitations. As most population-based surveys, we did not follow patients for 3 months to confirm CKD. This is necessary to diagnose CKD in the individual patient, but the implications of using a single determination for eGFR and albuminuria in epidemiological studies remain to be assessed. In addition, we did not include institutionalized population, who is usually old and sick; it may have underestimate the prevalence of CKD. Also, some selection bias cannot be ruled out, as the main reason for not participating in the study was to avoid a blood draw. Lastly, the cross-sectional design impeded to obtain causal conclusions on CKD correlates.
ConclusionThe prevalence of CKD in Spain was around 15%, a percentage far higher than previously reported in our country and comparable with figures in the United States. Also, there was a continuously increasing prevalence of CKD with the rising clustering of CVD risk factors, which suggests that CKD may be regarded as a quantifiable metric of cardiovascular risk. In addition, CKD could be considered as a cardiovascular condition in the population.
FundingThis study received funding from Fondo de Investigación Sanitaria (FIS) (Instituto de Salud Carlos III and FEDER/FSE) grants number 13/02321, PI16/01460. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gorostidi M, Sánchez-Martínez M, Ruilope LM, Graciani A, de la Cruz JJ, Santamaría R, et al. Prevalencia de enfermedad renal crónica en España: impacto de la acumulación de factores de riesgo cardiovascular. Nefrologia. 2018;38:606–615.