Death with a functioning graft (DWFG) is the most frequent cause of loss of kidney transplantation (KT).
ObjectiveTo analyze the evolution of the causes of DWFG and the frequency of the types of cancer causing DWFG.
MethodsRetrospective study of KT in Andalusia from 1984 to 2018. We analyzed the evolution according to eras (1984–1995; 1996–2007; 2008–2018) and according to post-transplant period (early death: first year post-KT; late death: after first year post-KT).
ResultsA total of 9905 KT were performed, registering 1861 DWFG. The most frequent causes were cardiovascular disease (25.1%), infections (21.5%) and cancer (19.9%).
In early death we did not observe changes, and infections were always the main cause. In late death, cardiovascular death decreased (1984−1995: 35.2%, 1996−2007: 22.6%, 2008−2018: 23.9%), but infections (1984−1995: 12.5%, 1996−2007: 18.3%, 2008−2018: 19.9%) and, above all, cancer-related deaths increased (1984−1995: 21.8%, 1996–2007: 29%, 2008−2018: 26.8%) (P < .001). In the multivariable analysis for late death due to cardiovascular disease, recipient age, retransplantation, diabetes, and the first period were risk factors, while the risk of late death due to cancer and infections was associated with recent eras.
In the first year after transplantation, the most frequent neoplasia causing DWFG was post-transplant lymphoproliferative disease, and after the first year, it was lung cancer, without differences when it was analyzed by eras.
ConclusionsDespite the greater comorbidity of the recipients, cardiovascular deaths have decreased. Cancer has been the main cause of late death in recent years. Lung cancer is the most frequent malignancy that causes DWFG in our transplant patients.
La muerte con injerto funcionante (MCIF) es la causa más frecuente de pérdida del trasplante renal (TR).
ObjetivoAnalizar la evolución de las etiologías de MCIF y la frecuencia de los tipos de neoplasia causantes.
MétodosEstudio retrospectivo de los TR en Andalucía desde 1984 hasta 2018. Analizamos la evolución de las MCIF según etapas (1984–1995; 1996–2007; 2008–2018) y según período post-TR (muerte precoz: primer año post-TR; muerte tardía: tras el primer año post-TR).
ResultadosSe realizaron 9.905 TR; se produjeron 1.861 MCIF. Las causas más frecuentes fueron enfermedad cardiovascular (25,1%), infecciones (21,5%) y neoplasias (19,9%).
En las muertes precoces no observamos cambios en el tiempo; las infecciones siempre fueron la causa principal. En las tardías, desciende la muerte cardiovascular (1984−1995: 35,2%; 1996−2007: 22,6%; 2008−2018: 23,9%) y aumentan las muertes por infecciones (1984−1995: 12,5%; 1996−2007: 18,3%; 2008−2018: 19,9%) y, sobre todo, por cáncer (1984−1995: 21,8%; 1996−2007: 29%; 2008−2018: 26,8%) (P < ,001). En el análisis multivariante para muerte tardía cardiovascular, edad del receptor, retrasplante, diabetes y primera etapa fueron factores de riesgo, mientras que el riesgo de muerte tardía por cáncer e infecciones se asoció con las etapas recientes.
La neoplasia más frecuente en el primer año post-TR fue la enfermedad linfoproliferativa post-TR y tras el primer año el cáncer de pulmón, sin diferencias entre etapas.
ConclusionesA pesar de la mayor comorbilidad del receptor, las muertes cardiovasculares han descendido. Las neoplasias son la principal causa de muerte tardía en los últimos años. El cáncer de pulmón es la neoplasia más frecuente causante de MCIF en TR.
Kidney transplant (KT) survival has improved significantly in recent years. The introduction of new, more potent and safer immunosuppression schemes has reduced the incidence of acute rejection.1,2 In the 1970s and early 1980s, the rejection rate was about 50% in the first year. Since the introduction of cyclosporine, this has been reduced by up to 15%, improving short-term results. Currently, the incidence of acute rejection in the first year is between 8 and 10% and graft survival in the first year is above 90% in most series.1,3,4
In the long term, we have also witnessed a notable improvement in results, largely due to continuous advances in surgical techniques, immunosuppression, and treatment of the various post-RT complications.1,5 The contributions in the field of immunology and the greater knowledge and characterization of histological lesions have also played a fundamental role. Advances in histological diagnosis have led to the development of new rejection classifications, have made it possible to discover renal involvement due to viral infections such as the BK virus and expand the study of recurrence of primary kidney disease in kidney grafts.1,6
This change of setting in KT has also determined that the causes of long-term graft loss have been modified in recent decades. Until the end of the 1990s, chronic rejection was still the main cause of graft loss after the first year of transplantation, accounting for more than 50% of the losses.2,3 At the beginning of the millennium, studies began to show that in the long term death with a functioning graft (DWFG) was the most frequent cause of graft loss. Several factors may have conditioned this change: a lower rate of graft loss, caused by the new immunosuppressants, and the older age of the patients undergoing KT with the presence of a greater number of comorbidities. It is therefore important to know the causes of death in our transplant recipients, since if we reduce the death rate among recipients with a functioning graft, the long-term survival of the renal graft will improve.7,8
Globally, the main cause of DWFG in patients with chronic kidney disease (CKD) has been cardiovascular disease.2,7,9 These patients have a high cardiovascular risk that may be due to pre-KT factors that sometimes also condition the evolution to terminal CKD, such as obesity, hypertension, hyperlipidemia, diabetes mellitus (DM) or smoking, as well as other post-KT factors such as immunosuppressive treatment.7 For all these reasons, it is widely established the need of evaluation of cardiovascular disease prior to RT and to vigorously treat those modifiable cardiovascular risk factors in order to reduce perioperative morbidity and mortality and after KT.7,9
However, the causes of DWFG also appear to have changed in recent years. In 2009, in a preliminary analysis of KT patients in Andalusia, we observed a tendency to decrease deaths of cardiovascular origin with an increase in mortality associated with cancer.10 Other large registries of kidney patients have also reported similar trends.8,11–14 The Andalusian Renal Transplant Registry (SICATA-TR) already has information in more than 10,000 KT performed and followed up for more than 35 years.15 In recent years, the characteristics of transplant patients have varied significantly, with an increase in age and comorbidities. For all these reasons, the main objective of this work is to update and analyze the evolution of the causes of DWFG in our country, throughout this long period, based on the data from this registry. As a secondary objective, we describe the frequency of the different types of neoplasia that cause DWFG in the KT patient.
MethodsIn Andalusia, since 1984, the data of all patients who have received hemodialysis, peritoneal dialysis or KT have been collected in a computerized registry. Patient variables include age, sex, cause of CKD, viral hepatitis serology, type and duration of previous renal replacement therapy (RRT), organ origin (deceased or living donor), combined solid organ transplant, length of survival and causes of graft failure and patient death according to the codes of the European Dialysis and Transplantation Association (EDTA).
We conducted a retrospective cohort study including all KT patients in Andalusia in the period between January 1, 1984 and December 31, 2018. Pediatric recipients and recipients of combined KT with other organs were excluded.
The causes of DWFG and its evolution over time were analyzed according to the stage in which the KT was performed: 1st period, 1984–1995; 2nd period, 1996–2007; 3rd period, 2008−2018. To make equal the follow-up in the different periods, DWFG after 10 years post-KT were excluded, since this is the maximum follow-up time of the third period. Likewise, DWFG were analyzed according to the post-KT period in which they occurred: early death was defined as that which occurred in the first year after KT, and late death as that which occurred after the first year after KT. In the case of DWFG due to cancer, we also analyzed the type of neoplasia and its distribution in relation to the time since the KT.
The EDTA codes for cause of death were grouped into 5 categories: cardiovascular death, infections, neoplasms, other, and unknown. Demographic variables, CKD etiology, hepatitis serology, DM as underlying renal disease, time and type of RRT, type of donor, retransplantation, and type of neoplasia were analyzed. Data were obtained from SICATA-RT and from the clinical records of hospitals that perform KT in Andalusia (Virgen de Rocío, Reina Sofía, Málaga Regional, Virgen de las Nieves and Puerta del Mar Hospitals).
Statistical analysisCategorical variables were expressed as frequency distribution and percentages, and continuous variables as the mean and standard deviation or as the median and interquartile range. The Kolgomorov-Smirnof test was used to assess the normality of the analyzed data. For the comparison of qualitative variables, the Chi-Square test was used and the continuous variables were compared using the Student’s T, Mann-Whitney U test, ANOVA or Kruskal-Wallis test, as appropriate. Bonferroni test was used for multiple comparisons of continuous variables.
Univariate and multivariate analyzes using Cox regression were performed to identify risk factors related to cardiovascular mortality, neoplasms, and infections in DWFG occurring after the first year post-KT. The multivariate analysis included those pre-KT variables with a known impact on patient survival, as well as the periods in which KT was performed. A value of P < .05 was considered significant. Statistical analysis was performed with SPSS for Mac version 25.0.
ResultsCharacteristics of all deceased recipients with functioning graft and their causes of deathDuring the study period, 10,902 patients were transplanted in Andalusia with a median follow-up until death or graft loss of 6.2 years [interquartile range 2.1, 12.2 years]. After excluding pediatric recipients (n = 518) and recipients of KT combined with other organs (n = 479), there were 9905 KT that met the inclusion criteria.
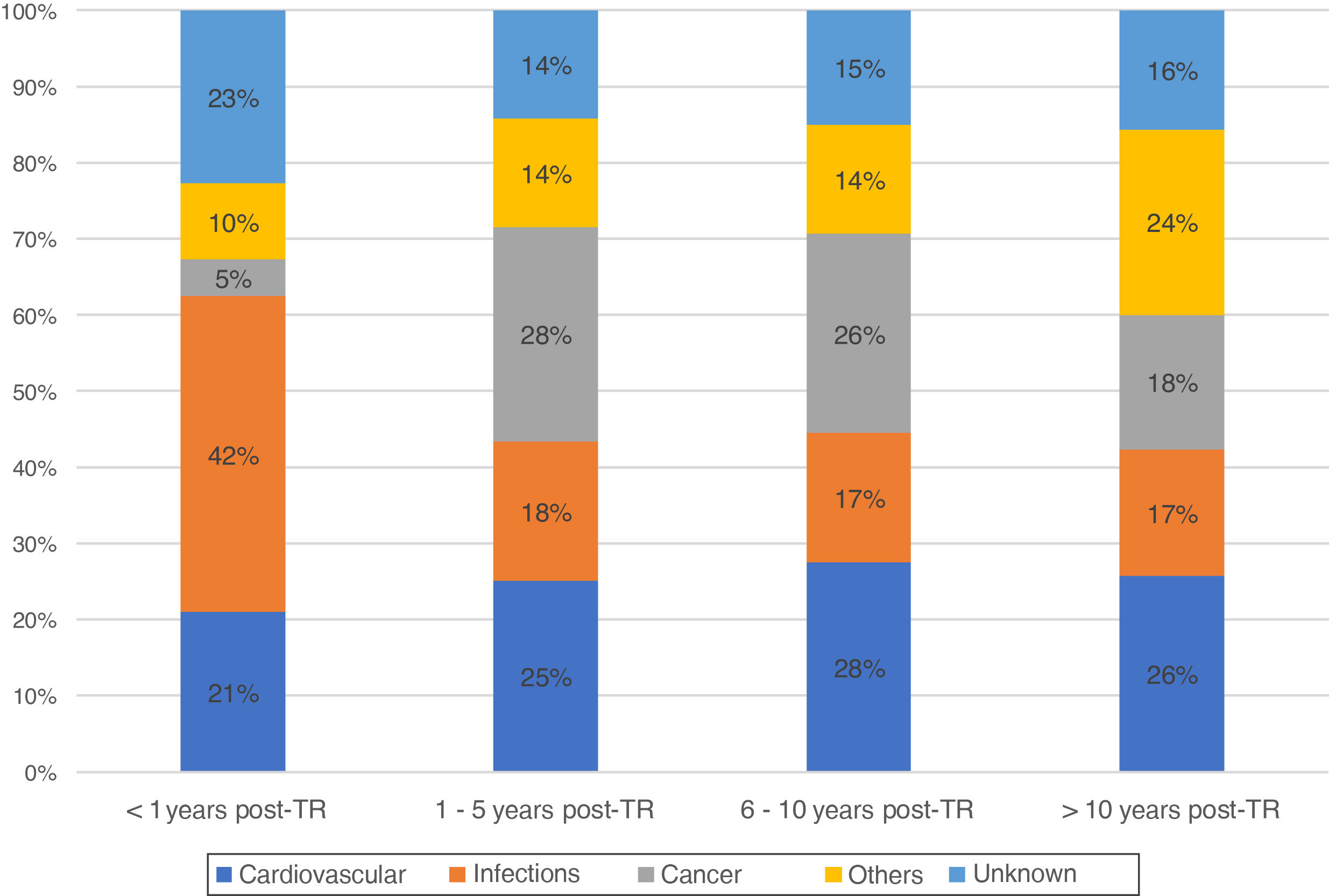
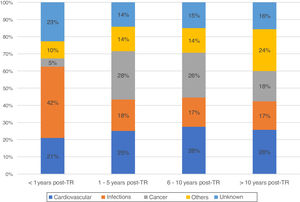
During this time, there were registered 1861 deceased patients with a functioning graft. The median time from transplant to death with a functioning graft was 6.8 years [2.3, 12.5 years]. Of these, 37% were women, with an average age of 54 years. The characteristics of the deceased recipients are shown in Table 1. The most frequent cause of DWFG was cardiovascular death (25.1%), followed by infections (21.5%) and neoplasms (19.9%). Infections were the most common cause of death in the first months after the transplant, with a very low incidence of cancer. Thereafter, cardiovascular events and neoplasms remain the main causes of DWFG (Fig. 1).
Clinical characteristics of the deceased patients with a functioning graft.
| Recipient age (years), mean (SD) | 53.96 (11.8) |
| Female sex, n (%) | 688 (37) |
| CKD etiology | |
| NAE, n (%) | 195 (10.5) |
| DM, n (%) | 183 (9.8) |
| GN, n (%) | 247 (13.3) |
| IN, n (%) | 249 (13.4) |
| ADPKD, n (%) | 241 (13) |
| Other hereditary, n (%) | 35 (1.8) |
| Others, n (%) | 278 (14.9) |
| Unknown, n (%) | 433 (23.3) |
| RRT pre-K mode | |
| HD, n (%) | 1560 (83.8) |
| PD, n (%) | 245 (13.1) |
| KT, n (%) | 56 (3) |
| Retransplantation, n (%) | 160 (8.5) |
| RRT time (months), median [IQR] | 30 [16.60] |
| HCV+, n (%) | 149 (8) |
| Cause of death | |
| Cardiovascular, n (%) | 468 (25.1) |
| Cardiac, n (%) | 311 (17.8) |
| Vascular, n (%) | 157 (8.4) |
| Infections, n (%) | 400 (21.5) |
| Cancer, n (%) | 370 (19.9) |
| Others, n (%) | 306 (16.4) |
| Unknown, n (%) | 317 (17) |
SD: standard deviation, CKD: chronic kidney disease, NAE: nephroangiosclerosis, DM: diabetes mellitus, GN: glomerulonephritis, IN: interstitial nephritis, ADPKD: autosomal dominant polycystic kidney disease, RRT: renal replacement therapy, HD: hemodialysis, PD: peritoneal dialysis, KT: kidney transplant, IQR: interquartile range, HCV: hepatitis C virus.
The characteristics of the recipients who died from any of these 3 main causes of death (n = 1238) were compared (Table 2). Cardiovascular disease was the first cause of death in both sexes (P < .001) and in diabetic patients (P = .012), as well as in those with prolonged time on RRT (P = .007).
Clinical and demographic characteristics of the deceased patients with a functioning graft according to the three main causes of death.
| Total | Cardiovascular | Infections | Cancer | P | |
|---|---|---|---|---|---|
| n (%) | 1238 | 468 (37.8) | 400 (32.3) | 370 (29.9) | |
| Age (years) of recipient at KT, mean (SD) | 54.2 (11.9) | 54.2 (11.4) | 54.7 (12.0) | 53.6 (12.3) | .478 |
| Female sex, n (%) | 454 (36.7) | 182 (40.1) | 168 (37) | 104 (22.9) | <.001 |
| DM, n (%)a | 128 (10.3) | 63 (49.2) | 38 (29.7) | 27 (21.1) | .012 |
| CKD etiology | |||||
| NAE, n (%) | 137 (11) | 46 (33.6) | 43 (31.4) | 48 (35) | .059 |
| DM, n (%) | 128 (10.3) | 63 (49.2) | 38 (29.7) | 27 (21.1) | |
| GN, n (%) | 168 (13.6) | 58 (34.5) | 60 (35.7) | 50 (29.8) | |
| IN, n (%) | 166 (13.4) | 64 (38.6) | 49 (29.5) | 53 (31.9) | |
| ADPKD, n (%) | 155 (12.5) | 63 (40.6) | 52 (33.5) | 40 (25.8) | |
| Other hereditary, n (%) | 23 (1.8) | 6 (26.1) | 9 (39.1) | 8 (34.8) | |
| Others, n (%) | 177 (14.3) | 52 (29.4) | 69 (39) | 56 (31.6) | |
| Nonaffiliated, n (%) | 273 (22.1) | 110 (40.3) | 75 (27.5) | 88 (32.2) | |
| Retransplantation, n (%) | 110 (8.9) | 50 (45.5) | 32 (29.1) | 28 (25.5) | .218 |
| RRT > 24 months, n (%) | 713 (57.6) | 273 (38.3) | 250 (35.1) | 190 (26.6) | .007 |
| Recipient age at death (years), mean (SD) | 61.9 (11.5) | 60.6 (11.5) | 59.7 (12.1) | 61.8 (11.1) | .094 |
KT: kidney transplant, SD: standard deviation, CKD: chronic kidney disease, NAE: nephroangiosclerosis, DM: diabetes mellitus, GN: glomerulonephritis, IN: interstitial nephritis, ADPKD: autosomal dominant polycystic kidney disease, RRT: renal replacement therapy.
For the analysis over time, as previously explained only DWFG occurred during the first 10 years post-KT were included, (n = 1290). Table 3 shows the characteristics of the patients with DWFG according to the period in which the KT was performed (1st period: 1984–1995, 2nd period: 1996–2007, 3rd period: 2008–2018). A progressive increase in the age of the recipient is observed, going from an average of 49 years in the first period to almost 63 in the last, which means an increase of 14 years in the age of access to KT (P < .001). The percentage of DM patients also increased significantly, representing 22.4% of the total number of patients who died with a functioning graft in the last period (P < .001). Likewise, the rate of retransplantation is higher in the recent periods (13.3% and 7.6% in the second and third periods, vs. 6.7% in the initial period; P = .001).
Clinical and demographic characteristics of the deceased patients with a functioning graft at the different periods of the study.
| 1984–1995 | 1996–2007 | 2008–2018 | P | |
|---|---|---|---|---|
| n (%) | 432 (27.9) | 527 (43.7) | 331 (24.3) | |
| Age of recipient at KT (years), mean (SD) | 48.9 (10.2)a | 57.2 (10.9)a | 62.8 (9.3)a | <.001 |
| Female sex, n (%) | 155 (35.9) | 192 (36.4) | 106 (32) | .387 |
| DM, n (%) | 25 (5.8) | 62 (11.8) | 74 (22.4) | <.001 |
| CKD etiology | ||||
| NAE, n (%) | 42 (10.1) | 56 (10.6) | 47 (14.2) | <.001 |
| DM, n (%) | 25 (5.8) | 62 (11.8) | 74 (22.4) | |
| GN, n (%) | 64 (15.5) | 63 (12) | 33 (10) | |
| NI, n (%) | 50 (12.1) | 65 (12.3) | 33 (10) | |
| ADPKD, n (%) | 55 (13.3) | 56 (10.6) | 36 (10.9) | |
| Other hereditary, n (%) | 8 (1.9) | 12 (2.3) | 4 (1.2) | |
| Others, n (%) | 65 (15.7) | 98 (17.5) | 33 (10) | |
| Unaffiliated, n (%) | 105 (25.4) | 121 (23) | 71 (21.5) | |
| Retransplantation, n (%) | 29 (6.7) | 70 (13.3) | 25 (7.6) | .001 |
KT: kidney transplant, SD: standard deviation, CKD: chronic kidney disease, NAE: nephroangiosclerosis, DM: diabetes mellitus, GN: glomerulonephritis, IN: interstitial nephritis, ADPKD: autosomal dominant polycystic kidney disease.
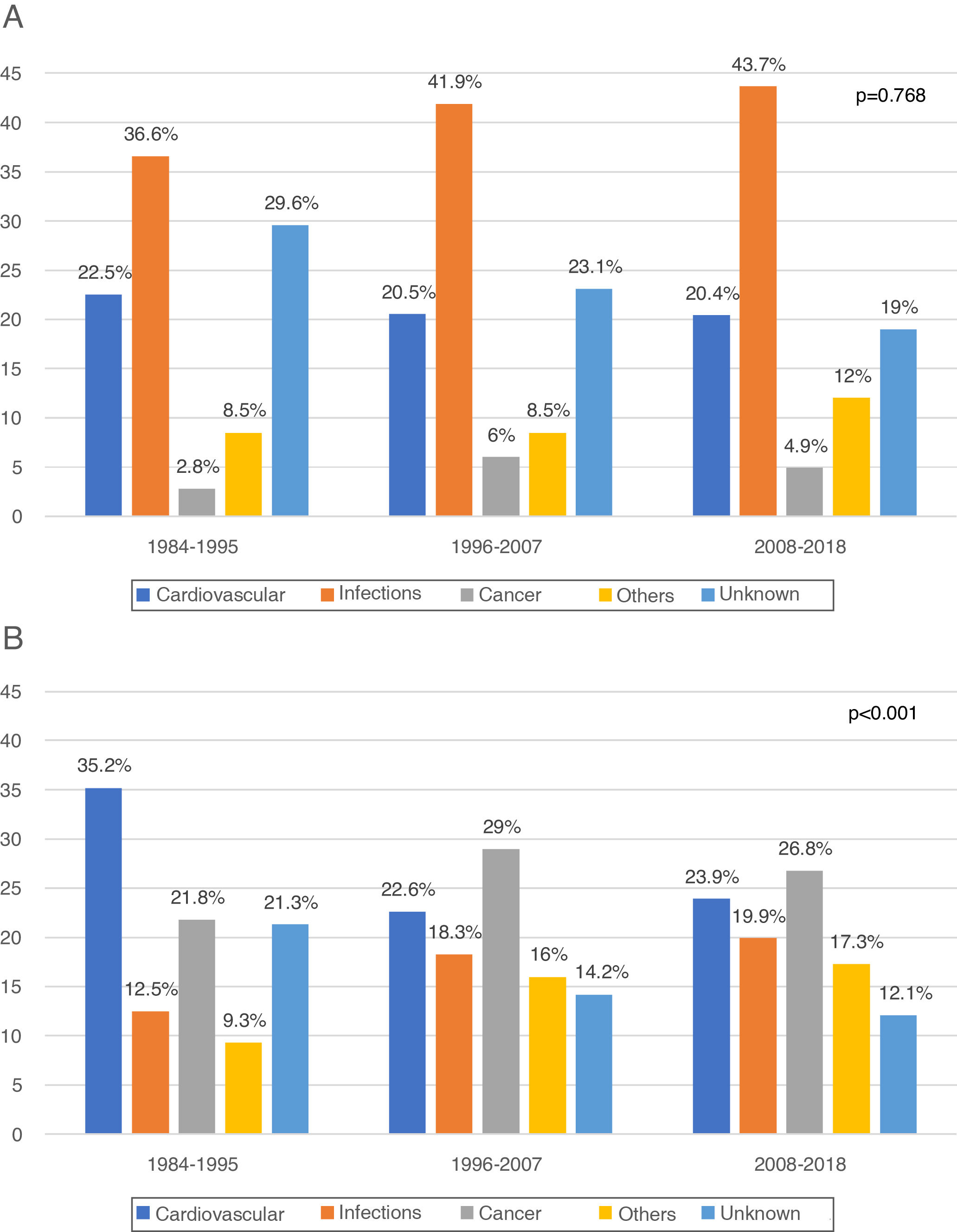
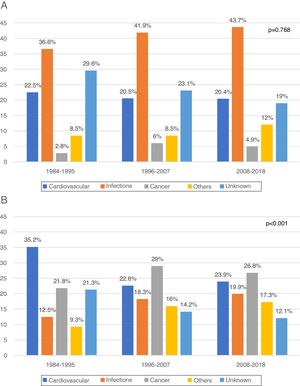
In the first year post-KT (early death) 330 DWFG were registered. After analyzing its evolution over time, we did not observe changes in the pattern of death between the different periods, infections always being the most frequent cause (1984−1995: 36.6%, 1996−2007: 41.9%, 2008−2018: 43.7%; P = .768) (Fig. 2a). The “other causes” variable includes a significant number of etiologies, with the greatest difference being observed in early DWFG in the decrease in deaths associated with graft hemorrhage (Table 4).
Evolution of early and late “other” causes of death in the different periods.
| 1984–1995 | 1996–2007 | 2008–2018 | ||||
|---|---|---|---|---|---|---|
| <1 year | >1 year | <1 year | >1 year | <1 year | >1 year | |
| Medullary aplasia, n (%) | 0 | 1 (0.8) | 0 | 0 | 0 | 0 |
| Chronic obstructive bronchopneumopathy, n (%) | 1 (3.2) | 4 (3) | 0 | 3. 4) | 0 | 0 |
| Cachexia, n (%) | 0 | 1 (0.8) | 0 | 2 (2.7) | 2 (9.1) | 0 |
| Non-viral cirrhosis, n (%) | 0 | 3 (2.3) | 0 | 3. 4) | 1 (4.5) | 0 |
| Liver failure, n (%) | 1 (3.2) | 4 (3) | 0 | 0 | 0 | 0 |
| Viral hepatitis, n (%) | 3 (9.7) | 28 (21.1) | 0 | 3. 4) | 0 | 1 (4.3) |
| Pancreatitis, n (%) | 1 (3.2) | 3 (2.3) | 1 (4.5) | 3 84) | 1 (4.5) | 3 813) |
| Perforation of Colon, n (%) | 0 | 2 (1.5) | 0 | 0 | 0 | 2 (8.7) |
| Peritonitis, n (%) | 1 83.2) | 2 (1.5) | 0 | 5 (6.7) | 1 (4.5) | 0 |
| Gastrointestinal bleeding, n (%) | 3 (9.7) | 9 (6.8) | 0 | 2 (2.7) | 2 (9.1) | 0 |
| Graft hemorrhage, n (%) | 14 (45.2) | 1 (0.8) | 8 (36.4) | 0 | 4 (18.2) | 1 (4.3) |
| Surgical bleeding, n (%) | 1 (3.2) | 2 (1.5) | 1 (4.5) | 1 (1.3) | 3 (13.6) | 1 (4.3) |
| Other bleeding, n (%) | 3 (9.7) | 4 (3) | 3 (13.6) | 3. 4) | 3 (13.6) | 1 (4.3) |
| Patient's Refusal to follow the treatment, n (%) | 0 | 1 (0.8) | 1 (4.5) | 1 (1.3) | 0 | 0 |
| Interruption of treatment for other reasons, n (%) | 0 | 1 (0.8) | 0 | 2 (2.7) | 0 | 0 |
| Accidental, n (%) | 2 (6.5) | 10 (7.5) | 4 (18.2) | 3. 4) | 0 | 0 |
| Dementia, n (%) | 0 | 9 (6.8) | 0 | 4 (5.3) | 0 | 0 |
| Suicide, n (%) | 0 | 6 84.5) | 0 | 5 (6.7) | 0 | 0 |
| Other causes, n (%) | 1 (3.2) | 42 (31.6) | 4 (18.2) | 35 (46.7) | 5 (22.7) | 14 (60.9) |
Comparison for the global etiologies included in “others”: P = .046 (early death); P = .019 (late death).
After the first year post-KT (late death) there were 960 DWFG. There is a reduction in deaths of cardiovascular origin (1984−1995: 35.2%, 1996−2007: 22.6% and 2008−2018: 23.9%) and a significant increase in deaths from cancer (1984−1995: 21.8%, 1996−2007: 29% and 2008−2018: 26.8%; P < .001), this being the most frequent cause of late death in the most recent periods (Fig. 2b). Although the number of deaths associated with viral hepatitis is low, there is even a clear decrease over time (Table 4).
In the multivariate analysis for late death of cardiovascular origin, the recipient's age, post-transplant status, and DM were independent risk factors, while only the 2nd period (1996–2007) behaved as a protector. For late death from neoplasia, DM was associated with a lower risk, while during the most recent period (2008–2018) the risk of death from cancer was increased. In the case of infections, the risk increased in the later periods (Table 5). After excluding diabetic patients in the multivariate analysis, the results for the 3 causes of death are similar in relation to the other variables (data not shown).
Multivariate Cox regression analysis of risk of late death from cardiovascular, infectious, and neoplasia.
| Cardiovascular death | Infectious death | Death from cancer | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Recipient age at TR | 1.01 (1.00–1.02) | .047 | 0.99 (0.97–1.01) | .241 | 1.00 (0.98–1.01) | .991 |
| Male sex | 1.02 (0.78–1.32) | .866 | 0.75 (0.55–1.03) | .079 | 0.78 (0.60–1.03) | .087 |
| DM | 1.58 (1.11–2.24) | .011 | 1.04 (0.66–1.64) | .853 | 0.64 (0.41 – 0.99) | .047 |
| Retransplantation | 2.18 (1.36–3.48) | .001 | 0.94 (0.48–1.81) | .849 | 1.11 (0.64–1.92) | .689 |
| RRT (months) | 1.00 (0.99–1.00) | .899 | 1.00 (0.99–1.00) | .958 | 0.99 (0.99–1.00) | .071 |
| Period (1996–2007)a | 0.53 (0.39 – 0.72) | <.001 | 1.53 (1.03–2.25) | .033 | 1.23 (0.91–1.68) | .170 |
| Period (2008–2018)a | 1.21 (0.81–1.83) | .341 | 4.34 (2.62–7.17) | <.001 | 3.14 (2.09–4.71) | <.001 |
KT: kidney transplant, DM: diabetes mellitus as a cause of chronic kidney disease, RRT: renal replacement therapy, HR: hazard ratio, CI: confidence interval.
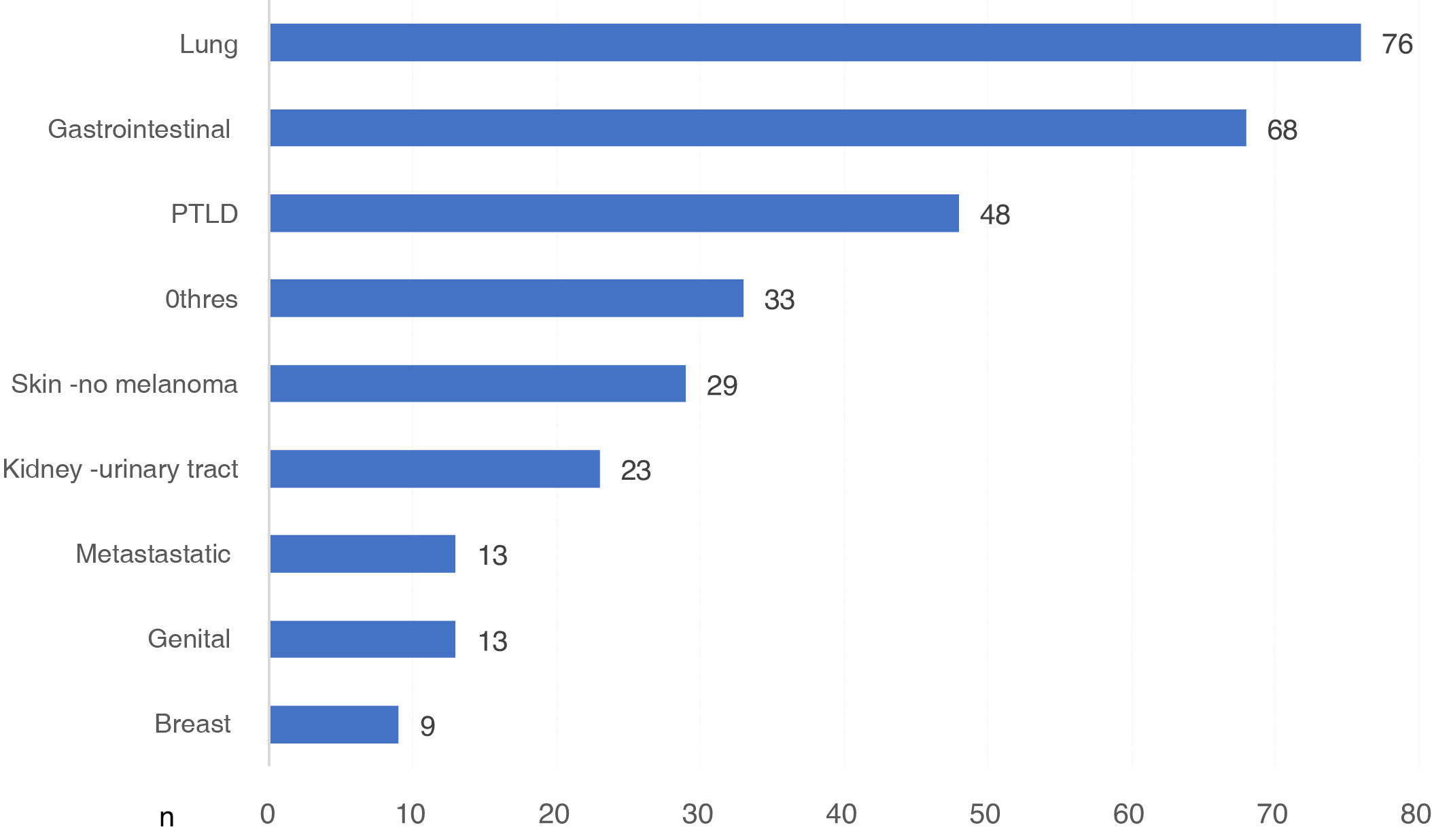
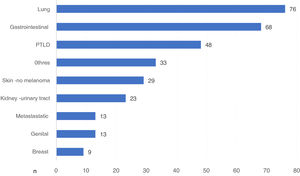
There were 370 DWFG secondary to cancer disease. We had the type of cancer in 312 cases, which represents 84.3% of those reported. Globally, lung cancer was the neoplasm that caused the most deaths, with 76 deaths, followed by G.I. cancer and post-KT lymphoproliferative disease (PTLD) (Fig. 3).
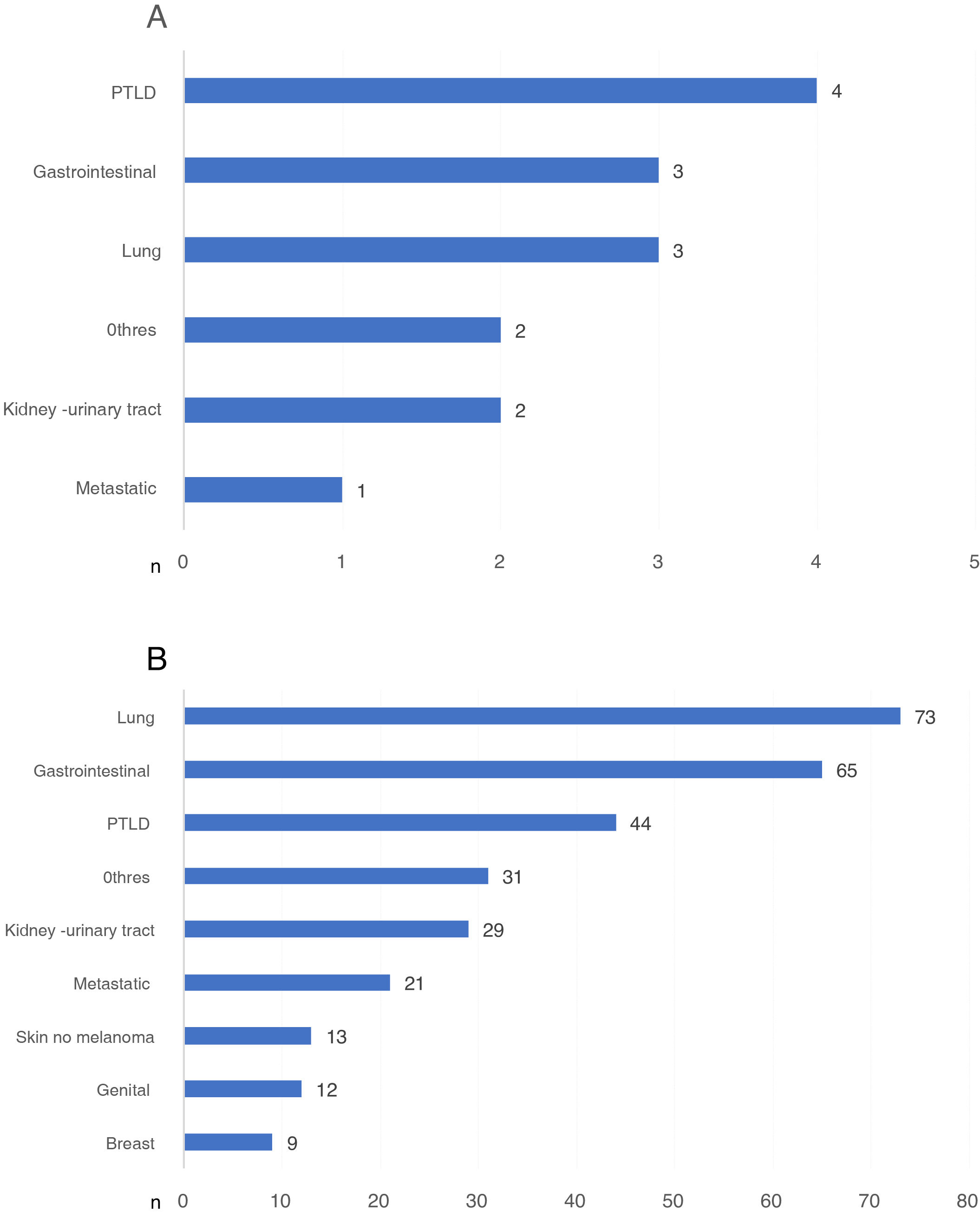
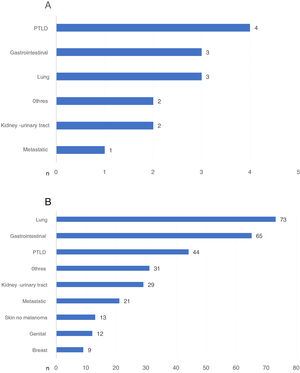
Those cancer-related DWFG that occurred in the first year post-KT were primarily due to PTLD (Fig. 4a). After the first year post-KT, lung cancer was the neoplasm that most frequently caused DWFG (Fig. 4b). We found no differences in the distribution of tumor types in the different stages (data not shown).
a) Types of neoplasm causing death with functioning graft in the first year post-transplantation, expressed in number of patients. b) Types of neoplasm causing death with functioning graft after the first year post-transplantation, expressed in number of patients.
PTLD: post-transplant lymphoproliferative disease.
We present the analysis of one of the largest series of deceased patients with a functioning graft. Our work confirms, in a large series with prolonged follow-up, the significant decrease in deaths of cardiovascular origin in recent decades, as well as the increase in deaths attributed to cancer in KT patients, which currently constitute the main cause of DWFG after first year post-KT in our experience. Likewise, we observe that, within the deaths due to neoplasia, lung cancer is the one that condition with the worst evolution, being the main cause of death due to tumor pathology.
We have observed the greatest differences in the causes of DWFG between the first period analyzed and the following ones. However, in the most recent periods the differences are smaller, as has been also observed in other geographical areas.13 In our case, for example, the difference in the age of the deceased is much greater between the first period and the second, than between this and the next period. It is possible that small demographic differences and similar immunosuppressive treatment regimens (more rational use of steroids, use of tacrolimus and mycophenolate) could explain, at least in part, the similar results observed in the last two periods evaluated.
Traditionally, the main cause of death in transplant patients has been cardiovascular disease.2,7,9 All patients with CKD have a high cardiovascular risk and, although in KT patients the risk is lower than in dialysis patients, it is still very high. It is estimated that KT have an annual rate of cardiovascular events of approximately 3.5%–5%.11,16–18 After transplantation, these cardiovascular risk factors are highly prevalent. Proof of this is that between 60%–80% of KT recipients have arterial hypertension, up to 30% suffer impaired glucose tolerance, 60% have dyslipidemia and approximately 20% are overweight.9,19,20 Although many of these factors are already present when the patient is on the waiting list, some factors related to KT are also added, such as chronic graft dysfunction. Likewise, immunosuppressive treatment can favor the development of cardiovascular risk factors or potentiate those already present before KT.9,18 All of this has been associated with higher mortality and worse long-term graft survival.19
In our case, both the age of the recipient and the DM were associated to a higher risk of cardiovascular death. Although it is reasonable to expect that age is associated with higher mortality in all areas, it is certainly considered the main risk factor for cardiovascular complications and death.21,22 A series of changes occur during aging, including an increase in the stiffness of the arterial tree, conditioning left ventricular hypertrophy, as well as deregulation of genes involved in oxidative stress, insulin signaling, and inflammation.21–23 All of this leads to a greater risk of ischemia and heart failure, which could justify the fact that more than 30% of deaths in people over 65 years of age are due to cardiovascular disease.23 A Spanish study analyzed mortality from acute myocardial infarction stratified by age, observing a progressive increase with age, going from 19% in the group of less than 65 years to 84% in the group of more than 85 years.24
As for DM, it is also a known cardiovascular risk factor. The mortality risk of diabetic patients is the same as that of a patient who has suffered a myocardial infarction.25 These data have led to the fact that DM is currently considered one of the main cardiovascular risk factors and it is not different for KT patients.26 It is estimated that the risk of cardiovascular death is 10 times higher than in non-diabetic patients, including those who develop DM post-KT.27,28 In our experience, in addition to being a risk factor for cardiovascular death in the multivariate analysis, it behaved as a protector with respect to death from cancer, most likely reflecting the competing risk between the two, as has already been reported by others.29,30
As a result of the abovementioned, the clinical practice guidelines strongly recommend treating cardiovascular risk factors in KT patients.17,31 Likewise, it is common practice to exhaustively study and treat cardiovascular disease in patients who access KT.2,7,9 Consequently, it is likely that the generalization of all these recommendations may explain, at least in part, the decrease in recent years in deaths caused by cardiovascular disease in KT, as reflected in the data provided by different registries, such as our case.8,11–13
We observed this trend in DWFG that happened after the first year post-KT. The fact that patients who underwent a transplant between 1996 and 2007 presented lower cardiovascular mortality may reflect the influence of primary prevention strategies, but it is possible that changes in other transplant-specific factors may also have had an influence such as the introduction of immunosuppression schemes more safe and with less side effects. Ciclosporin has been widely replaced by tacrolimus, which has less potential to cause hypertension or dyslipidemia.32 In adition, the tendency to minimize steroids reduces the probability of DM, among other beneficial effects.12,25 Thus, despite the progressive increase in the age and comorbidity of patients included on the waiting list, as confirmed by the data presented, the lowest rate of deaths from cardiovascular causes has also been maintained in recent years, which seems to reflect a positive stabilization of this trend.
Parallel to what has been observed in relation to cardiovascular death, neoplasms seem to have been gaining prominence as a cause of DWFG in the long term.11–14 It is estimated that patients who receive solid organ transplants have a risk of developing cancer between 2 and 4 times higher than the general population adjusted for age and sex, and this risk seems to increase with the time post-KT.33 In addition to the classic risk factors, such as age, sex, race or exposure to toxins, there are also those risk typical of transplantation, such as immunosuppression, time on dialysis and the influence of certain oncogenic viruses.29,34–36 For some authors, this change in trend may be due to a decrease in cardiovascular disease rather than to an increase in tumors.12 However, the worse evolution of cancer in transplant recipients has been clearly established, as well as its relationship with increasingly powerful immunosuppressive regimens in recent years due to the increasing complexity of the receptors, which could directly contribute to the relevance of neoplasms as a cause of death in this population.29,34–36
The role of age as a risk factor for the development of cancer is well defined in the general population.37 However, the relationship between age and cancer as a cause of death is controversial in KT. The absolute risk of developing cancer is higher in older transplant patients. However, the relative risk is much higher in young transplant recipients due to the rarity of cancer in the general population at younger ages.29 Despite the fact that some studies point to age as a risk factor for death from neoplasia in transplant recipients, in our registry the multivariate analysis did not demonstrate this association.38 Other authors also discuss the direct effect of age on the increase in deaths from cancer in KT patients and, as in DM, consider that it is more likely related to competitive risk with other causes of death, including cardiovascular.29,30
In a similar manner to what we observe in transplant patients, in the general population we have also been observing a change in the causes of death for some years now. In the United States, deaths due to heart disease were almost double than those caused by cancer in the 1980s, while in recent years they have clearly tended to be equal.39 In our country, according to the annual report of the Ministry of Health, malignant tumors caused more than 25% of the total documented deaths, which is more than deaths of cardiac and vascular origin.40 Therefore, in addition to transplant-specific factors, general factors (more heart-healthy habits, decreased smoking, exposure to toxins, greater diagnostic capacity for tumors…) could also be influencing the trend in the causes of death observed in our countries, transplanted.
Additionally, our work offers information on the type of cancer in patients who died from neoplasia. In our experience, PTLD is the neoplastic pathology that produced most death in the early post-RT period. Although cancer is a rare cause of death in recent post-KT, PTLD has a bimodal distribution, characteristically showing a higher incidence peak in the first year which is associated with high mortality, which supports our data and may explain that it is the most common cause of cancer death in this early period.41
However, both globally and in the long term, lung cancer is the neoplasia that most frequently caused death in our patients. Again these findings are not specific to the transplant population. In the latest report on the number of cancer in Spain in 2020 published by the Spanish Society of Medical Oncology, lung cancer is the tumor that caused the highest number of deaths in our country, as in the rest of the world.42,43 One possible explanation for this is that its course is usually silent and its diagnosis is made in advanced stages.44 Furthermore, although the risk of lung cancer is only slightly increased in KT patients compared to the general population, their mortality is significantly higher.45–47 Unfortunately, we do not have data on the incidence of smoking in our population to be able to analyze its relationship with the development of this neoplasm. Some groups recommend annual screening by means of low-radiation chest CT in KT patients with a higher risk of developing lung cancer (history of smoking or active smokers), although it remains to be established whether this practice can be extended and improve the prognosis of this cancer in our patients.29
Apart from the two main causes of late DWFG, cardiovascular and cancer, previously discussed, we have observed other changes over the years. The growing experience of surgical teams and advances in the knowledge and treatment of viral hepatitis are most likely reflected in a decrease in DWFG associated with these causes.
In relation to death caused by infectious, the risk of late death due to infection possibly increases due to multiple factors described in the literature, such as the increase in the immunosuppressive potency of current therapies, the age of the recipients, and the incidence of infections by multi-resistant germs, among others.48
Our work has limitations. First, it is a retrospective analysis with all the limitations inherent to this design. Also, we only have a limited number of variables that helps us to explain the observed changes of mortality over time, without data on some factors closely related to mortality such as smoking, hypertension, ischemic heart disease, peripheral vascular disease or dyslipidemia. Furthermore, we did not have data on the type of immunosuppression; however, to minimize this limitation, the periods were established by their temporal relationship with changes in immunosuppression: from the pre-tacrolimus era (first period), to the extended use of tacrolimus and mycophenolate (second period) and, finally, to the use very common in induction therapies (third period). Likewise, the analysis has been adjusted for post-KT time to eliminate, as far as possible, the bias that the time factor has on the causes of death, thus the time of follow-up was limited to 10 years post-KT in all periods. Finally, we cannot know the influence that the COVID pandemic may have had on the causes of death of our patients. We believe that this should be the subject of a specific analysis and for this reason we have not extended the time of study that we are presenting now.
ConclusionsIn conclusion, we can state that the evolution of the causes of death in the KT patient follows a pattern similar to that of the general population. Deaths of cardiovascular origin have decreased significantly and it has been maintained in recent years despite the greater age and comorbidity of the recipients. By contrast, neoplasms are now the main cause of late death in recent years. In the first year, PTLD is the neoplasia that most frequently causes death and later on the lung cancer becomes the main cause of death by tumors. Globally, lung cancer, as in the general population, is the neoplasm with the worst prognosis, which should be taken into account when establishing follow-up plans and protocols for our transplant patients.
FundingThis research has not received specific funding from public sector agencies, the commercial sector, or non-profit entities.
Conflict of interestThe authors declare that they have no conflicts of interest.