Cardiovascular (CVD) and chronic kidney disease (CKD) in women have unique risk factors related to hormonal status and obstetric history that must be taken into account. Pregnancy complications, such as preeclampsia (PE), can reveal a subclinical predisposition for the development of future disease that may help identify women who could benefit from early CVD and CKD prevention strategies.
Materials and methodsReview of PE and its association with future development of CVD and CKD.
ResultsMultiple studies have established an association between PE and the development of ischemic heart disease, chronic hypertension, peripheral vascular disease, stroke and CKD. It has not been sufficiently clarified if this relation is a causal one or if it is mediated by common risk factors. Nevertheless, the presence of endothelial dysfunction and thrombotic microangiopathy during pregnancies complicated with PE makes us believe that PE may leave a long-term imprint. Early identification of women who have had a pregnancy complicated by PE becomes a window of opportunity to improve women’s health through adequate follow-up and targeted preventive actions. Oxidative stress biomarkers and vascular ultrasound may play a key role in the early detection of this arterial damage.
ConclusionsThe implementation of preventive multidisciplinary targeted strategies can help slow down CVD and CKD’s natural history in women at risk through lifestyle modifications and adequate blood pressure control. Therefore, we propose a series of recommendations to guide the prediction and prevention of CVD and CKD throughout life of women with a history of PE.
La enfermedad cardiovascular (ECV) y renal en la mujer presentan factores de riesgo propios relacionados con el estatus hormonal y los antecedentes obstétricos que deben tenerse en cuenta. Las complicaciones del embarazo, como la preeclampsia (PE), pueden revelar predisposiciones subclínicas a padecer enfermedades futuras que ayuden a identificar a aquellas mujeres que puedan beneficiarse de nuevas oportunidades para la prevención de la ECV y la enfermedad renal crónica.
Materiales y métodosRevisión sobre la PE y su asociación con el desarrollo de ECV y renal futuras.
ResultadosMúltiples estudios han establecido una asociación entre PE y el desarrollo de cardiopatía isquémica, hipertensión crónica, enfermedad vascular periférica, accidente cerebrovascular y enfermedad renal. No se ha aclarado suficientemente si esta relación es de causalidad o está mediada por la presencia de factores de riesgo comunes. Sin embargo, la demostración de fenómenos de disfunción endotelial y microangiopatía trombótica en los embarazos que cursan con PE hace suponer que esta puede dejar una impronta a largo plazo. La identificación precoz de las mujeres que han padecido un embarazo complicado con PE es una ventana de oportunidad para mejorar la salud de la mujer, mediante su seguimiento y la adopción de medidas preventivas adecuadas. Los marcadores bioquímicos de daño oxidativo y la ecografía vascular pueden desempeñar un papel clave en la identificación precoz de este daño arterial.
ConclusionesLa implantación de estrategias preventivas multidisciplinares y específicas puede ayudar a frenar la historia natural de la ECV y renal en las mujeres de riesgo, a través de la modificación de su estilo de vida y del adecuado control de la tensión arterial. Para ello, proponemos una serie de recomendaciones para guiar el estudio de la predicción y prevención de la ECV tras la PE a lo largo de la vida de la mujer.
Cardiovascular disease (CVD) is the leading cause of death in women, with a mortality rate of 49% in Europe, 16 times higher than that of breast cancer (3%).1 This generates a great economic and social burden, as well as significant physical and psychological consequences for women and their families. Although 80% of CVD events are preventable, it is estimated that the mortality rate in women is increasing, especially at younger ages.2 Despite this, the importance of CVD in women is underestimated because of a lack of social awareness, due to the erroneous perception that they are protected against it. Consequently, preventive resources for CVD in women are not sufficiently prioritized.
There are several underlying reasons:
- 1)
Traditionally, women have been excluded from clinical trials, wrongly assuming that cardiovascular risk factors (CVR) and recommendations should be similar for men and women.3
- 2)
CVR stratification is inadequate. Most women younger than 60 years are classified in the low-risk category for CVD, even in the presence of high CVR factor burden.4
- 3)
CVD risk in women is influenced by specific factors such as early menopause or a history of pregnancy complications such as preeclampsia (PE), gestational diabetes, or preterm delivery. These factors are not included in the traditional CVR assessment.5
- 4)
The clinical manifestations of CVD in men and women show differences that lead to the fact that cardiovascular disease and its severity are being under-recognized in women.
Symptoms of CVD in women are often more complex and multifactorial than in men,6 which, together with lack of educative information, leads to women to consulti at more advanced stages. In summary, all these observations highlight the need for a female-oriented approach, including specific risk factors and intervention programs. In this review, we highlight the timing of pregnancy, and especially the development of PE, as a marker of cardiovascular health.
Pregnancy as a new paradigm for the identification of cardiovascular risk in womenPregnant women, their families and health personnel usually approach pregnancy as a transitory situation. Once the new mother is discharged, the focus shifts to the newborn. If there was a complication during pregnancy (e.g., PE), surveillance is usually maintained until the signs and symptoms of illness disappear, with the expectation that this will restore the woman’s health. Concerns about its impact on future health are often limited to the potential effects on subsequent pregnancies, and its long-term influence is forgotten or unknown.7 However, as we will see, PE affects a woman’s health for the rest of her life.
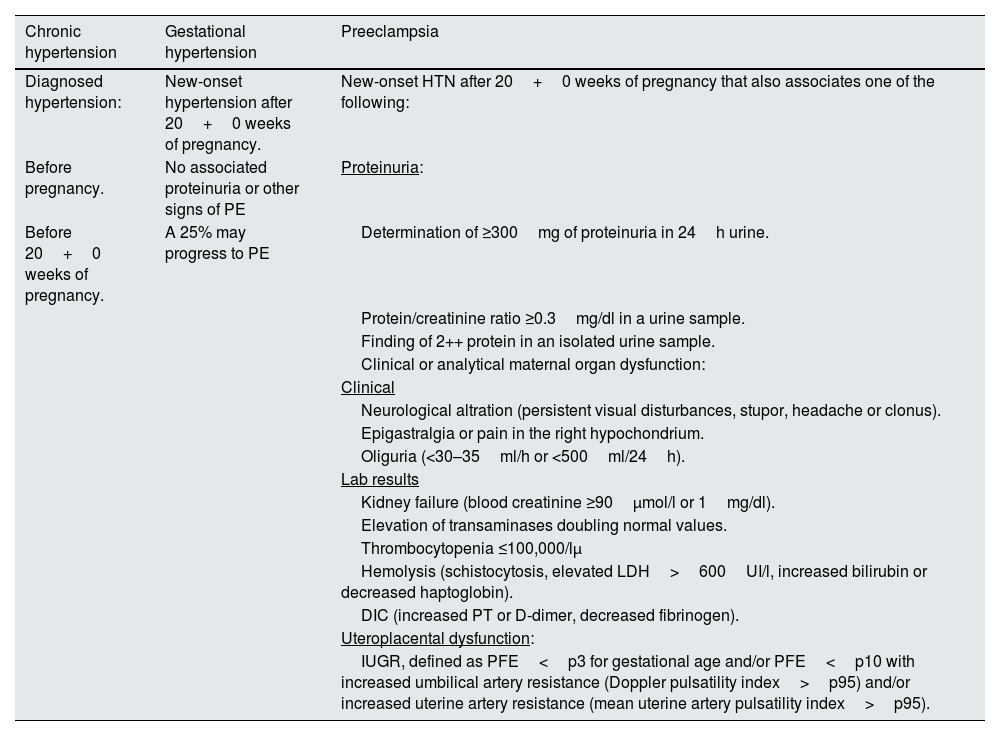
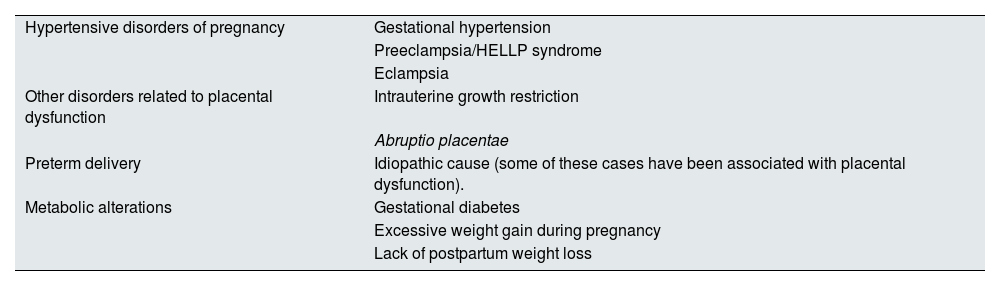
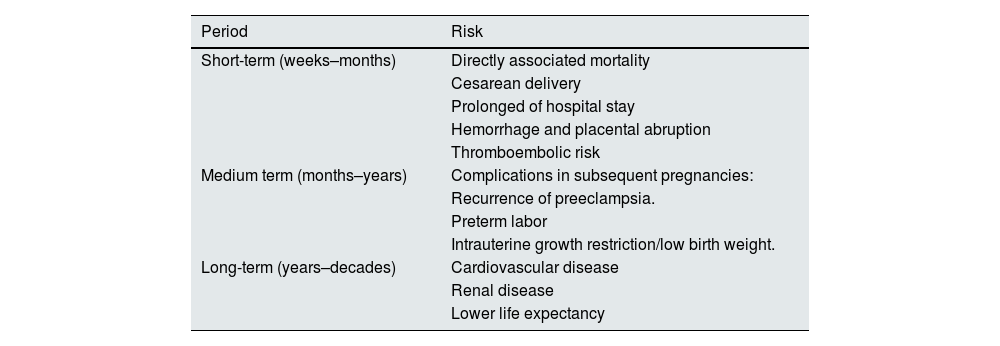
PE and other hypertensive disorders of pregnancy (Table 1) affect 10% of pregnant women. PE is at the top of the list of causes of maternal mortality, accounting for approximately 1 in 6 deaths in pregnant women8 and the leading cause of admission to intensive care units during the puerperal period.9 Although hypertension and other clinical symptoms usually resolve in the weeks following delivery, its biological effects are maintained over time, predisposing to insulin resistance, hypertension, endothelial dysfunction and, ultimately, metabolic syndrome.10 PE forms part of some gestational alterations that have been shown to be associated with future CVR in women, such as other hypertensive states of pregnancy, gestational diabetes or preterm delivery (Table 2). Of these, the most consistent association is shown by PE.11 CVD and PE share risk factors, such as hyperlipidemia, obesity and hypertension itself, and PE may act as a factor reinforcing the high baseline risk of these patients.12 PE has also been shown to increase the risk of renal disease and complications in subsequent pregnancies. Table 3 summarizes its complications for the mother in the short, medium and long term.
Differential diagnosis of hypertensive disorders of pregnancy.
| Chronic hypertension | Gestational hypertension | Preeclampsia |
|---|---|---|
| Diagnosed hypertension: | New-onset hypertension after 20+0 weeks of pregnancy. | New-onset HTN after 20+0 weeks of pregnancy that also associates one of the following: |
| Before pregnancy. | No associated proteinuria or other signs of PE | Proteinuria: |
| Before 20+0 weeks of pregnancy. | A 25% may progress to PE | Determination of ≥300mg of proteinuria in 24h urine. |
| Protein/creatinine ratio ≥0.3mg/dl in a urine sample. | ||
| Finding of 2++ protein in an isolated urine sample. | ||
| Clinical or analytical maternal organ dysfunction: | ||
| Clinical | ||
| Neurological altration (persistent visual disturbances, stupor, headache or clonus). | ||
| Epigastralgia or pain in the right hypochondrium. | ||
| Oliguria (<30–35ml/h or <500ml/24h). | ||
| Lab results | ||
| Kidney failure (blood creatinine ≥90μmol/l or 1mg/dl). | ||
| Elevation of transaminases doubling normal values. | ||
| Thrombocytopenia ≤100,000/lμ | ||
| Hemolysis (schistocytosis, elevated LDH>600UI/l, increased bilirubin or decreased haptoglobin). | ||
| DIC (increased PT or D-dimer, decreased fibrinogen). | ||
| Uteroplacental dysfunction: | ||
| IUGR, defined as PFE<p3 for gestational age and/or PFE<p10 with increased umbilical artery resistance (Doppler pulsatility index>p95) and/or increased uterine artery resistance (mean uterine artery pulsatility index>p95). |
CID: disseminated intravascular coagulation; IUGR: intrauterine growth restriction; LDH: lactate dehydrogenase; p: percentile; PE: preeclampsia; EFW: estimated fetal weight; PT: prothrombin time; HTN: hypertension.
Pregnancy-related cardiovascular risk factors for which scientific evidence is at least level II-2.
| Hypertensive disorders of pregnancy | Gestational hypertension |
| Preeclampsia/HELLP syndrome | |
| Eclampsia | |
| Other disorders related to placental dysfunction | Intrauterine growth restriction |
| Abruptio placentae | |
| Preterm delivery | Idiopathic cause (some of these cases have been associated with placental dysfunction). |
| Metabolic alterations | Gestational diabetes |
| Excessive weight gain during pregnancy | |
| Lack of postpartum weight loss |
Women’s health risks associated with the development of preeclampsia.
| Period | Risk |
|---|---|
| Short-term (weeks–months) | Directly associated mortality |
| Cesarean delivery | |
| Prolonged of hospital stay | |
| Hemorrhage and placental abruption | |
| Thromboembolic risk | |
| Medium term (months–years) | Complications in subsequent pregnancies: |
| Recurrence of preeclampsia. | |
| Preterm labor | |
| Intrauterine growth restriction/low birth weight. | |
| Long-term (years–decades) | Cardiovascular disease |
| Renal disease | |
| Lower life expectancy |
Source: Shih et al.63
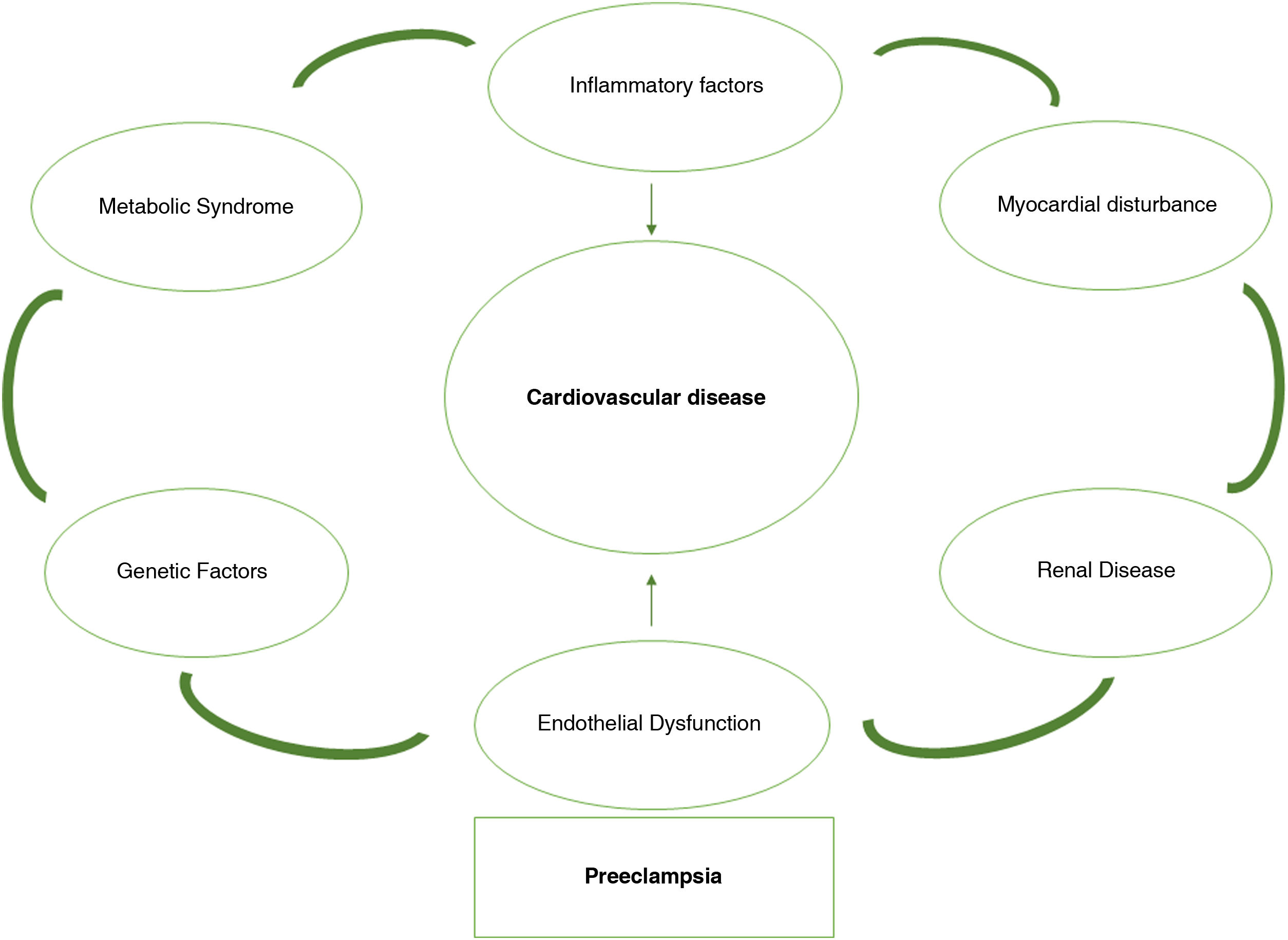
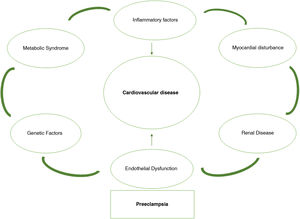
Indeed, in recent years the concept has spread that pregnancy complications, such as PE, may reveal subclinical predispositions to future disease that help to identify those women who may benefit from new opportunities for chronic disease prevention.13 Pregnancy proves to be a cardiovascular stress test. Some pregnant women may exceed the limit of vascular dysfunction and develop PE, which demonstrates their vulnerability to recurrence of PE and later CVD. The frequency with which a woman fails this exercise stress test and the severity of this failure marks the future risk of CVD (Fig. 1). Following this argument, if we can identify these women at risk after a first complicated pregnancy with a PE, we can implement early screening programs to modify lifestyle habits or initiate treatments that can mitigate the risks in subsequent pregnancies and also for long-term cardiovascular health.14 Therefore, we are faced with the possibility of converting a problem into a real window of opportunity to improve women’s health.
Concept of placental dysfunction and its pathogenic relationship with short- and long-term endothelial injuryIn recent years there have been significant advances in the understanding of the pathogenesis of placental dysfunction, which is responsible of multiple closely linked complications of pregnancy, especially EP, constrained intrauterine growth and abruptio placentae. In addition, it is also associated with preterm delivery and intrauterine death.15
The etiology of placental dysfunction is multifactorial. There are involved various genetic, environmental and immunological factors, most CVR factors (dyslipidemia, hypertension, obesity, diabetes mellitus) and other abnormalities such as systemic lupus erythematosus, thrombophilias and nephropathies. Apart from its ultimate origin, it is presently established as “angiogenic hypothesis” that links placental dysfunction with the damage that appears in the maternal endothelia through an imbalance in the processes of angiogenesis. Thus, in response to situations of placental hypoperfusion or hypoxia, the placenta releases into the maternal circulation an excess of the anti-angiogenic factor known as the soluble form of tyrosine kinase-1 similar to fms (sFlt-1, for soluble fms-like tyrosine kinase-1), which blocks the endothelial regenerating action of proangiogenic factors such as placental growth factor (PlGF) and vascular endothelial growth factor. This angiogenic imbalance triggers a generalized endothelial dysfunction in the pregnant woman that causes the onset of hypertension, and cause damage to the fenestrated endothelia of the target organs affected by PE, such as the brain, liver and kidney.16
In the second half of pregnancies complicated by placental dysfunction, an elevated sFlt-1/PlGF ratio can be detected in maternal serum at least one month before the first clinical signs and symptoms appear. Moreover, the sFlt-1/PlGF alteration is greater in the most severe and early cases. Its main clinical application is to guide in situations of suspected preterm PE, with a high negative predictive value for the appearance of PE in the following week (99%) and in the following 4 weeks (94%) when the sFlt-1/PlGF ratio is ≤38.17 However, when the pregnancy is at term (37–42 weeks), PE usually presents with milder forms and with less alteration of the angiogenic balance.18,19 In these cases of late PE, placental abnormalities are is less marked and frequent. On the contrary, its onset is usually the result of a combination of maternal predisposing factors and worse cardiovascular function secondary to the cardiovascular overload of pregnancy, which reaches its peak at this time. This ultimately compromises placental perfusion, finally leading to the onset of PE.20
According to this model, 2 different hypotheses can be put forward to justify the endothelial damage and predisposition to CVD and renal disease in women who have suffered a gestational complication related to placental dysfunction. According to a first hypothesis, this risk would be conditioned by the sequelae of angiogenic imbalance produced during pregnancy and could affect women without pregestational risk factors. Angiogenic imbalance not only cause an endothelial alteration, but also other immunoinflammatory abnormalities that play a long-term role in the cardiovascular health of women, and it is not clear whether this is a permanent condition in the mother (and even in her children) or whether it can be reversed over the years.21,22 Additionally, sympathetic hyperactivity, decreased plasma volume and decreased baroreflex sensitivity, which have also been observed in the postpartum period, have been postulated as autonomic alterations that may be the cause of subsequent arterial hypertension.23 In the second hypothesis, both placental dysfunction and future CVD would be conditioned by pre-existing CVR factors, without the influence of angiogenic imbalances in pregnancy.24 It is expected that new long-term follow-up and intervention studies, which take into account the role of angiogenic imbalance in pregnancy, will clarify the true causal relationship between placental dysfunction and CVD.
Long-term risks following preeclampsia and other complications related to placental dysfunctionCardiovascular complicationsIt has been known since the 1960s that PE is a risk factor for the development of chronic hypertension,25 but its association with long-term CVR has not been specifically evaluated until the last decade.26 In 2011, the American Heart Association included in its cardiovascular prevention guidelines the evaluation of women’s obstetric history, where the presence of hypertensive disorders of pregnancy is a major factor in CVR, recommending close follow-up in these women.27
Multiple studies have established a clear association between PE and CVD, however, the origin of PE is still unclear.28 Both PE and atherosclerosis include in their pathophysiology the activation of monocytes and inflammatory factors that result in endothelial dysfunction. Within pregnancy itself, there are also a series of factors that modulate the risk of subsequent development of CVD, with a higher risk in women in whom PE develops at a younger gestational age, has a more severe course, and has at least one normal first pregnancy, with a cumulative risk.7
The reported impact of PE on CVD varies across studies, as follow-up periods differ and different definitions are used for both PE and the set of entities that comprise CVD. In Appendix A, the Supplementary Table 1 summarizes the main studies.
The relative risk (RR) of developing chronic hypertension in the future varies between 3.1 and 3.7,12 with a mean follow-up after delivery of 14 years. Women who have had PE in 2 or more pregnancies have up to a 10-fold increased risk of requiring antihypertensive medication in the future.29
The risk of CVD and infarction in women with a history of PE has been confirmed in multiple epidemiological studies. The RR of ischemic heart disease is 1.5–2.2, after 10 years of delivery.30
It has also been observed that surrogate markers of CVD, such as the coronary artery calcification index, are significantly elevated in women with a history of PE when compared to healthy women 30 years after the index pregnancies.31,32
Additional components of CVD, such as heart failure, peripheral artery disease, and CVD mortality, double their risk,33 with a significant increase in death from stroke also observed in patients with a history of PE.34 Likewise, a history of PE increases the risk of death in patients with coronary revascularization, with an adjusted RR of 1.6.35
The risk of developing diabetes mellitus in women with a history of PE is as much as double,36 which may further increase the CVR of these patients.
In short, PE is a major risk factor for the development of ischemic heart disease, chronic hypertension, peripheral vascular disease, stroke and diabetes mellitus and this is underestimated in routine clinical practice.
Renal complicationsPE has been defined as a disease with transient and reversible renal involvement within 3 months after delivery. In addition, women with chronic kidney disease (CKD) have a significantly increased risk of developing PE. PE nephropathy is considered to be a glomerular endotheliosis that can lead to glomerular dysfunction expressed as proteinuria and podocyturia.37 It is usually unknown whether the endothelial lesions that develop during PE reverse after delivery; certainly a renal biopsy is usually not performed after delivery. It is also unknown whether sFlt-1 levels, and other markers that are altered in PE, such as inflammatory cytokines and angiotensin II type 1 receptor agonist autoantibodies, remain altered to any degree after delivery. On the other hand, the imbalance in the organization of the podocyte cytoskeleton could also cause a loss of podocytes in the urine and compensatory glomerular hypertrophy, which leads to changes in the glomerular basement membrane, an increase in the extracellular matrix and the development of focal glomerulosclerosis that ends up in the appearance of proteinuria and CKD.38–40
There is little information in the literature on the risk of developing CKD after PE.41 A systematic review of retrospective studies highlighted the association between PE and persistent microalbuminuria after delivery. After a median follow-up of 7.1 years after delivery, 31% of women with PE developed microalbuminuria compared to 7% of women with uncomplicated pregnancies. Women with severe PE had an 8-fold increased risk of developing microalbuminuria. There were no differences in serum creatinine values or renal glomerular filtration rate.42 However, the inclusion criteria of this study required a minimum follow-up of only 6 months after delivery, which is too short a period to assess the risk of CKD or advanced chronic kidney disease (ACKD). The results reported by other studies with longer-term follow-up have been more contradictory. Classic studies directly linked PE with a 4-fold increased risk of developing ACKD within 10 years after pregnancy.43 However, the Dutch Prevention of Renal and Vascular End-stage Disease – PREVEND – study, which analyzed renal function in women with a history of hypertensive disorders during pregnancy, did not observe increased proteinuria or incidence of CKD.44
To better assess the long-term renal risk of PE, Covella et al. conducted a systematic review including studies with a follow-up of at least 4 years. This review highlighted that the RR of developing ACKD was significantly higher40 in 110,803 women with PE compared to 2,680,929 women as a control group. However, they were unable to elucidate whether the risk of ACKD was related to the presence of underlying diseases that might predispose to the development of PE or to the severity of PE itself. In parallel to what happens with other CVD, there are important limitations in these retrospective studies to identify the phenotype of the patient with PE who is more at risk of developing ACKD, since the risk factors for CKD and PE overlap.45–47
Finally, in recent years several studies have shown association of pregnancy with various forms of thrombotic microangiopathy, including atypical hemolytic uremic syndrome. These describe a new view of the involvement of dysregulation of the alternative complement pathway in the etiopathogenesis of atypical hemolytic uremic syndrome and a form of PE known as hemolysis, elevated transaminases and thrombocytopenia syndrome (HELLP). In this sense, complement should be considered as a new protagonist in vascular disease due to its direct involvement with the endothelium, and thus, its alterations should be considered as a new CVR factor in pregnant women.48,49
Early detection of subclinical atherosclerosis and preventive strategies in women with a history of preeclampsiaAtherosclerosis remains one of the most important public health problems for women in the western world. Atherosclerosis is influenced by the presence of CVR factors, including PE, and it is critical to identify strategies for its prevention and early diagnosis. Imaging can play a key role by identifying atherosclerotic plaques at early stages. According to clinical practice guidelines, the most appropriate techniques are coronary calcium quantification by CT and vascular ultrasound. The latter makes it possible to identify the presence of atherosclerotic plaques in the vascular wall in a simple, non-invasive and rapid manner. It has also been shown that ultrasound detection of plaque is associated with the occurrence of CVD,50 with the same accuracy as coronary calcification detected by CT.
A population-based study The Progression of Early Subclinical Atherosclerosis – PESA – has been studying for the past 10 years more than 4000 individuals aged 40–54 years with imaging techniques to evaluate the presence and progression of atherosclerosis in early stages. It has been observed that nearly 50% of women aged 40–54 years have subclinical atherosclerosis, and 25% have 2 or more affected arterial territories.51 Even in the absence of CVR factors, 40% of the participants had subclinical disease.52 However, all of them were at low risk according to the European SCORE. This lack of accuracy of the scores in predicting CVD in women could be related to the lack of analysis of their specific CVR factors.
There is ample evidence demonstrating the benefit of CVD prevention at early stages. However, it has not been systematically implemented, which contrasts with the widespread development of breast cancer screening, despite the fact that CVD mortality in women is much higher than breast cancer. Significant progress has been made in raising awareness of the importance of CVD in American women through initiatives such as Go Red for Women, or the Mujeres por el Corazón campaign of the Spanish Society of Cardiology, whose main objective is to raise awareness among women about CVD prevention and promote healthy habits.
Proposal for the comprehensive care of women who have suffered preeclampsiaAs has been explained, PE is a risk factor of CV and Renal disease that, once identified, should lead to early intervention to halt the development and progression of these diseases. As a starting point, it is essential to highlight the importance of a correct identification of those women who develop PE during pregnancy. To this end, it is necessary to insist on correct blood pressure (BP) measurement during obstetric visits, often limited by lack of time, lack of appropriate equipment or incorrect practices (such as, for example, the use of incorrectly sized cuffs, BP measurement in the recumbent position, or taking only one measurement). Particular attention should be paid to blood pressure values, comparing them whenever possible with the references from the first trimester and their trajectory throughout gestation.53 If an increasing trend is observed, signs or symptoms of PE should be monitored, and proteinuria should be determined (an isolated determination with protein/creatinine index is valid). However, it is very important to bear in mind that PE does not always present with hypertension and proteinuria, but sometimes has more heterogeneous forms of presentation. It is therefore essential to always maintain a high degree of suspicion,54 and in these cases the angiogenic biomarkers sFlt-1-PlGF can be used to confirm or rule out the diagnosis of PE.
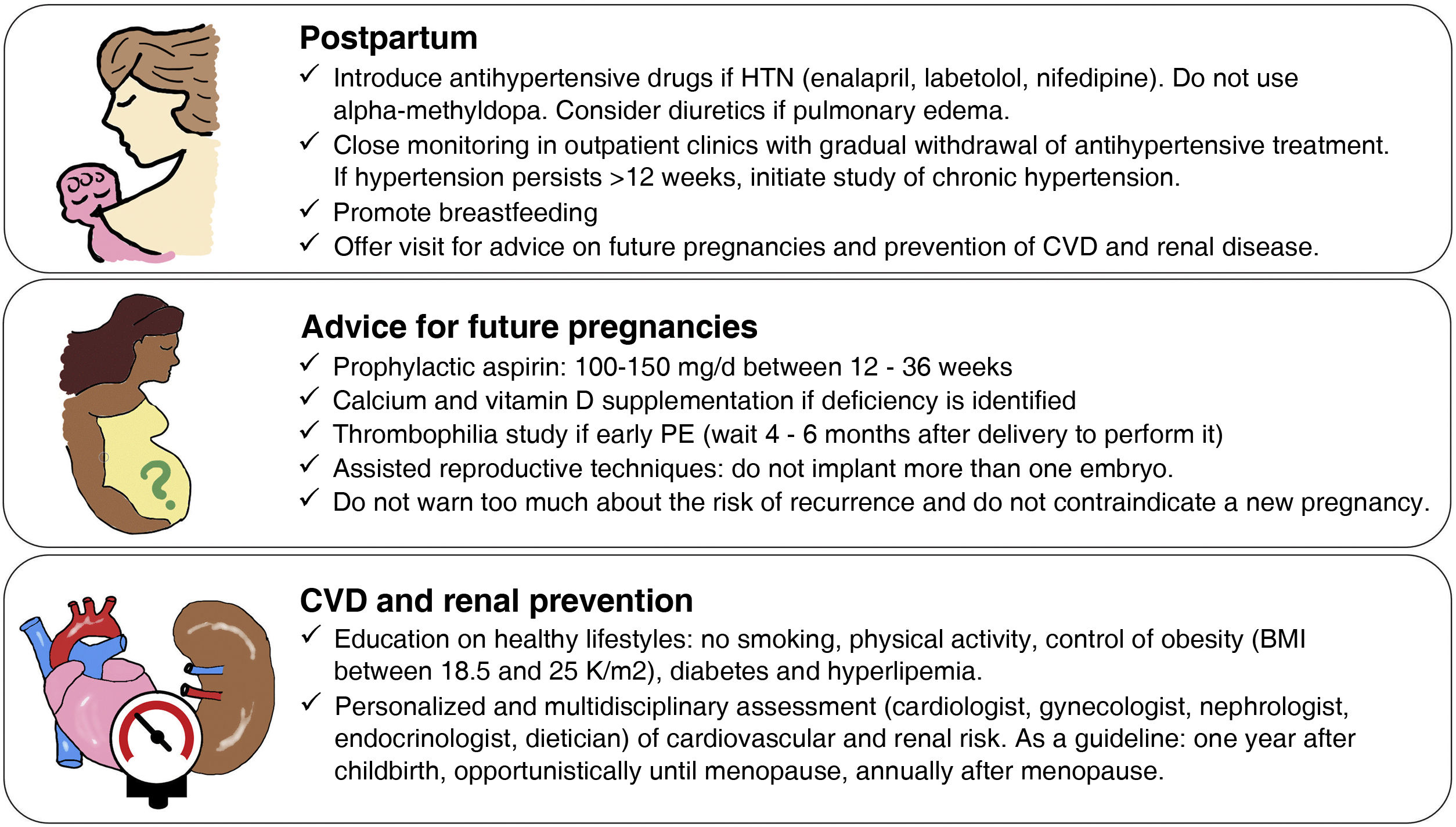
We propose a follow-up strategy that begins in the postpartum period and will accompany the woman throughout her life. It is based on the one we have implemented in recent years for the follow-up of cases of early PE, involving obstetricians, nephrologists and cardiologists (Fig. 2).
First, unified strategies for the management of hypertension and thromboembolic risk in the puerperium should be established. Obstetricians, nephrologists, and primary care physicians should carefully follow women presenting with postpartum hypertension and be familiar with both antihypertensive treatments compatible with breastfeeding and the need for thromboprophylaxis. In general, it is recommended to introduce antihypertensive medication whenever postpartum BP is >140/90mmHg,55,56 and drugs such as enalapril, nifedipine or labetalol can be used in the first line, avoiding alfamethyldopa because of its association with puerperal depression. Close ambulatory monitoring of BP evolution should be carried out, which in most cases will allow the gradual withdrawal of antihypertensive medication. Those postpartum women in whom hypertension persists despite the use of antihypertensive drugs at maximum doses will require complementary studies and a multidisciplinary approach with nephrologists, anesthesiologists and endocrinologists to establish a differential diagnosis. The risk of venous thromboembolism is increased in the puerperium (0.3/1000 pregnancies) and even more after PE (1.2/1000 pregnancies).57 There is no consensus among the different clinical guidelines on how to perform thromboprophylaxis. Our proposal is to use low molecular weight heparin in prophylactic doses during the first 2 weeks of puerperium after EP and increase to 6 weeks in the case of early EP (before 34 weeks) or after EP and cesarean section.58
Secondly, it is advisable to offer preconception counseling within the first year after the EP episode or when the patient requests it, since many women who have had an EP often have doubts and uncertainty regarding their reproductive future. The risk of recurrence of PE in subsequent pregnancies is approximately 15%, although it may be higher if it has occurred in more than one pregnancy, if it resulted in preterm delivery, or if there are predisposing conditions.59 Although these figures may be discouraging, it should be emphasized that in most subsequent pregnancies PE does not occur or does not manifest severely and that the prophylactic use of low-dose aspirin (150mg/d taken at night between 12 and 36 weeks of gestation) prevents more than 60% of early forms of PE.60 In those women with a history of early PE it is advisable to perform a thrombophilia study in case they could also benefit from prophylactic anticoagulation. Although it has been proposed to prevent PE recurrence through lifestyle changes, there are still important gaps in knowledge about their possible benefit.61 Nevertheless, it seems reasonable to recommend healthy lifestyle habits and referral to a specialist if CVR factors such as hypertension, obesity, smoking, diabetes or dyslipidemia are detected.
Finally, the challenge of long-term CVD prevention needs to be addressed. Given that CVD can take decades to manifest after PE, the question is: when should screening and intervention be initiated? In our opinion, up to the age of 50 years, an opportunistic approach could be taken by primary care physicians and obstetrician-gynecologists, taking advantage of visits for other causes to evaluate CVR factors. After the age of 50 years, it is advisable to carry out a specific assessment of CVR and renal disease, implement appropriate prophylactic or therapeutic measures, and establish annual or case-specific follow-up visits (Fig. 2).
ConclusionsPregnancy is a cardiovascular stress test that provides valuable information on the woman’s future CVR. Although no clear causality has been established between PE or other situations of placental dysfunction and the development of ischemic heart disease, chronic hypertension, peripheral vascular disease, stroke, or kidney disease, the association between them is undeniable. Therefore, it is essential to raise awareness among both patients and professionals of the future risks and to support the creation of multidisciplinary programs for the adequate follow-up and prevention of CVD and renal disease in women with this obstetric history.
FinancingThe present work has been funded by the Instituto de Salud Carlos III with the project “Cardiovascular health in women with a history of early preeclampsia” (grant PI19/01579), and by the Spanish Society of Cardiology, through a Grant for Translational Research Projects in Cardiology (grant TP18/0308).
Conflict of interestThe authors declare that they have no conflicts of interest.
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access
- Léalo en español
- Download PDF
- Bibliography
- Additional material