The basilic vein is a deep vein which usually requires superficialisation and surgical transposition.
Material and methodsThis is a retrospective study of 119 BBAVF-ST in patients with stage 5D chronic kidney disease who received an implant with a one-stage surgical technique (2011–2015). The percentage of primary (PP), assisted primary (APP) and secondary (SP) permeabilities were assessed, as well as the related complications. We analyzed the permeabilities using Kaplan–Meier survival curves and a univariate Log Rank analysis (Mantel–Cox). p values less than or equal to 0.05 were considered as significant.
ResultsThe mean age of the study group was 67.9years, with 63.8% of the subjects being male. A total of 57 complications were detected during the follow-up period: 24 stenosis (42.1%), 11 thrombosis (19.2%), 7 vascular access steal syndromes (12.2%), 7 upper limb oedemas (12.2%), 6 post-puncture haematomas (10.5%) and 2 infections (3.5%). The percentages of PP obtained at 1, 6, 12 and 24months were 92.4%, 79.8%, 66.3% and 52%; APP: 94.1%, 87.3%, 80.4% and 65.6%, and SP: 95%, 89.1%, 84% and 67.5%, respectively. Diabetic patients presented with significantly worse permeabilities than vascular or idiopathic patients: (p=.037, .009 and .019, respectively).
ConclusionsAccording to the results obtained in our study, the one-stage surgical implementation of BBAVF-ST presents high permeability rates and a small number of related complications. Diabetes mellitus is a factor related to a worse surgical prognosis. Some of the biggest advantages are the greater optimization of health resources and a shorter time in which the central venous catheter needs to remain in the body.
La vena basílica se caracteriza por ser un vaso profundo que en la mayoría de los casos requiere superficialización y trasposición quirúrgica.
Material y métodosEstudio retrospectivo de 119 FAVn HB S-T en pacientes con insuficiencia renal crónica 5D implantadas en un solo acto quirúrgico (2011-2015). Se analiza el porcentaje de permeabilidades primaria (PP), primaria asistida (PPA) y secundaria (PS), así como las complicaciones asociadas. Análisis de permeabilidades mediante curvas de supervivencia Kaplan-Meier y análisis univariante mediante Log Rank (Mantel-Cox). Se considera significativa una p≤0,05.
ResultadosEdad media 67,9años y 63,8% hombres. Durante el período de seguimiento se objetivaron un total de 57 complicaciones: 24 estenosis (42,1%), 11 trombosis (19,2%), 7 síndromes de robo vascular (12,2%), 7 edemas de extremidad superior (12,2%), 6 hematomas pospunción (10,5%) y 2 infecciones (3,5%). Los porcentajes de PP obtenidos a 1, 6, 12 y 24meses: 92,4, 79,8, 66,3 y 52; PPA: 94,1, 87,3, 80,4 y 65,6%, y PS: 95, 89,1, 84 y 67,5%. Se constataron diferencias significativas en las curvas de PP, PPA y PS según la etiología, presentando peores permeabilidades los diabéticos respecto a la vascular e idiopática (p=0,037, 0,009 y 0,019).
ConclusionesLa implantación quirúrgica de FAVn HB S-T en un solo acto ofrece buenas tasas de permeabilidad y escaso número de complicaciones asociadas. La diabetes mellitus representa un factor de peor pronóstico quirúrgico. Entre las mayores ventajas destacan una mejor optimización de los recursos sanitarios y menor tiempo de permanencia del catéter venoso central.
Different studies have shown that hemodialysis patients with native arteriovenous fistulas (NAVF) have more dose of dialysis, a long period of patency and less complications.1–3 By contrast, patients with central venous catheters show worse survival curves, due to a greater morbidity and mortality due to infections.4–6
According to the recommendations of the current Spanish Vascular Access Guidelines,7 the placement of an AVF as distal as possible should be the first surgical option. However, in some patients in whom the placement of a radiocephalic or humerocephalic AVF is not feasible, the placement of more proximal accesse should be a third or fourth surgical option as an alternative to the placement of a vascular prosthesis.8
Dagher et al.9 were the first surgeons to describe in 1976 the use of this type of AVF as vascular access for patients in regular hemodialysis. The basilic vein is characterized by having of good caliber but in most cases it is deep when placed in a deep plane to the aponeurotic tissue. In addition, this vascular structure runs adjacent to the vascular and nervous bundle of the arm, so superficialization of the vein and its transposition is recommended to move away from the surgical wound, avoiding fibrosis and facilitating its cannulation after completion of the maturation process.
In the current vascular guidelines, there is no consensus to recommend implantation in one or two surgical procedures.7,10,11 The main advantage of a single step procedure is to shorten the time required to start the cannulation and the possibility to remove the central venous catheter. However, in some cases, mechanical complications during the surgical procedure could occur with the mobilization of a non-arterialized vein. Some authors claim that, if performed in two stages, the vein could have increased the length which facilitate superficialization with less intraoperative complications. This second stage forces either to ligate the previous anastomosis and do it again after superficialization, or to cut sensitive branches. nervous that run over the basilic vein.
The main objective of the present study is to analyze our experience in the surgical placement of humerobasilic nFAV with superficialization and transposition in a single surgical act.
ObjectivesPrimary objective. To analyze our results in the implantation of humero-basilic nAVF with superficialization and transposition in a single surgical act: patency rates (primary, primary assisted and secondary) and associated complications during the follow-up period. Secondary objective. Study possible relationships between patency rates and different sociodemographic or comorbidity factors.
MethodsThe Functional Unit of Vascular Access of the Hospital Clínic is a transversal and multidisciplinary structure for the management of vascular access of patients in different areas of Catalonia (nephrology, vascular surgery and angiorradiology). All endovascular and surgical procedures are recorded in our database. The present study, is an observational and retrospective analysis of 119 humerobasilic fistulas that were superficialized and transposed in a single surgical act (HB ST nAVF) out of a total of 1.676 interventions performed between 2011 and 2015. Te follow-up period lasted until December 31, 2017; transplant patients and those who have died were collected. Different variables were recorded: (a) Cardiovascular risk (hypertension, diabetes, ischemic heart disease, dyslipidemia, cerebrovascular accident or peripheral vascular disease); (b) Antiplatelet or anticoagulant treatment; (c) Presence of ipsilateral central venous catheter or contralateral pacemaker, and (d) Pre-surgical echographic parameters (diameter of basilic vein and humeral artery). Each patient was evaluated in our outpatient clinic by tdifferent specialists including an exhaustive and systematic physical examination and eco-Doppler (mapping). The HBV S-T nAVF was performed in patients with no possibility to have distal (radio-cephalic) accesses because of an arterial and or vein diameter <2mm or more proximal (humerocephalic) due to a sclerosed vein or with a diameter <3mm. In these cases, if the proximal basilic vein is permeable, with enough caliber (>3mm) and well connected with the deep venous system, as well as a suitable artery (greater than 2.5cm in diameter) diameter and with three-phase Doppler curve, we chose to perform this type of access, and only occasionally forearm prosthetic loops.
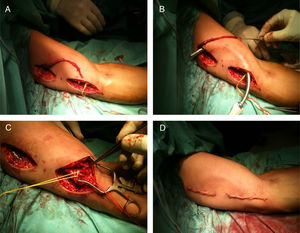
Normally the HB S-T nAVF is performed as an outpatient. The patient comes to the hospital the same day of surgery, and is discharged 1–2h observation post-surgery. The surgical procedure is performed under regional anesthesia, by echo guided axillary plexus blockade. Briefly, the surgical act consists in dissecting the basilic vein by means of two longitudinal incisions in the anterior aspect of the arm (instead of a long one, to minimize the complications of the wound). The basilic vein is tunneled through the anterior side of the arm making a curve (to avoid fibrosis of the surgical wounds on the vein), after demonstration of its permeability with physiological saline solution, the vein is anastomosed end-to-side with the humeral artery. The presence of pulse and thrill in the vein is confirmed intraoperatively, as well as its correct permeability by Doppler ultrasound and the absence of distal ischemic events. After a brief observation period (1–2h) the patient is discharged and monitored on an outpatient basis. Fig. 1 shows each of the surgical steps graphically.
Surgical procedure of implantation of a native humerobasílica FAV with superficialization and transposition in a single surgical act. Dissection of the basilic vein by means of two complementary incisions (A), tunneling in the anterior subcutaneous plane (B), arteriovenous anastomosis (C) and final appearance after closure of surgical wounds (D).
- -
Primary permeability (days). It is defined as the period of time elapsed since the placement of the HB S-T nAVF and the need to apply any endovascular or surgical technique to maintain the permeability of the vascular access.
- -
Assisted primary permeability (days). It is defined as the period of free time elapsed since the placement of the HB S-T nAVF and the appearance of thrombosis.
- -
Secondary permeability (days). It is defined as the period of time elapsed since the surgical placement of the HB S-T nAVF and the loss of vascular access (thrombosis not recanalizable). Transplanted patients, those lost in follow-up and those who died have been considered as censored cases for the three permeabilities.
- -
Initial surgical success. HB S-T nAVF functioning with manifest pulse, continuous murmur and presence of thrill after completion of the surgical procedure.
- -
Adequate maturation. It is defined by clinical and ultrasound criteria as described by latest Spanish Vascular Access Guidelines.7
The statistical analysis included descriptive techniques, chi-square test for proportions and Student's t for continuous variables, using the statistical package SPSS v21 (IBM Corp, Armonk, NY). Kaplan–Meier survival curves were used to estimate the rates of primary, assisted primary and secondary patency during follow-up. An univariate analysis was performed using Log Rank analysis (Mantel–Cox) to compare how permeabilities are affected by main prognostic factors of permeability (sex, age over 70 years, etiology, hypertension, dyslipidemia, ischemic heart disease, antiaggregant treatment or of previous central venous catheter); variables present in less than 5% of the sample (pacemaker, anticoagulation, peripheral vasculopathy, stroke) and in those with subcategories with less than 5% representation (etiology) were excluded from the analysis. The etiologies were grouped as diabetic, vascular, idiopathic etiology and other etiologies. The permeabilities estimated at 2 years are described. A p≤0.05 is considered significant.
ResultsA total of 119 patients with chronic kidney failure stage 5D were included, all patients had the placement of HB S-T nAVF. Mean age of 67.9±14.2 years (21–91), 63.8% were males. The causes of kidney failure were diabetes mellitus (31.1%), vascular disease (46.2%) and idiopathic (15.1%). A surgical success was achieved in 97.4% of the patients and adequate maturation was observed in 89.1%. Table 1 summarizes the demographic and clinical description of the patients included. During the follow up period, 12 patients were transplanted and 11 died (all of them with functioning fistulas), and the follow up was not completed in 26 patients and were considered losses. The mean(SD) follow-up was 888 (601)days. During the follow-up period, there were a total of 57 complications: stenosis n=24 (42.1%), thrombosis n=11 (19.2%), vascular steal syndromes n=7 (12.2%), edema of the upper extremity n=7 (12.2%), post-puncture hematomas n=6 (10.5%) and infections n=2 (3.5%). Regarding the location of the stenosis, in 9 cases affected the first portion of the efferent vein that were treated with 8 angioplasties and one surgical re-anastomosis), 9 cases in the first venous portion and the middle third (8 angioplasties and one surgical repair), 4 cases in the innominate trunk (4 angioplasties) and 2 at the level of the proximal one third and axillary vein (2 angioplasties).
Main sociodemographic and comorbidity factors of the study group.
| Variable | n and (%) or mean±SD (range) |
|---|---|
| Sex (males/females) | 76/43 |
| Average age (years) | 67.9±14.2 (21–91) |
| Hypertension | 96 (81%) |
| Mellitus diabetes | 37 (31%) |
| Dyslipidemia | 44 (37%) |
| Ischemic heart disease | 14 (11.8%) |
| Vascular-cerebral accident | 6 (5%) |
| Peripheral vascular disease | 2 (1.7) |
| Antiaggregant treatment | 25 (21%) |
| Anticoagulant treatment | 5 (4.2%) |
| Previous central venous catheter | 14 (11.8%) |
| Pacemaker | 3 (2.5%) |
| Previous FAVn, mean (range) | 2.4 (1–4) |
| Diameter basilic vein (mm) | 4.01±1.00 (2–7.5) |
| Diameter humeral artery (mm) | 3.9±0.8 (2.4–8) |
The percentages of primary permeability (PP) estimated with Kaplan Meier survival curves, at 1, 3, 6, 12, 18 and 24hr months were 92.4, 89.1, 79.8, 66.3, of 57.3 and 52.0%. The assisted primary permeability (APP) was 94.1, 92.4, 87.3, 80.4, 73.0% and 65.6%, and the percent of secondary permeability (SP), were 95.0, 93.3, 89.1, 84.0, 75.9 and 67.5%, respectively.
Comparison of permeability curves (PP, PPP and PS) during follow-up showed that among the possible prognostic factors (sex, age over 70 years, etiology, hypertension, dyslipidemia, ischemic heart disease, antiplatelet therapy or use of a previous central venous catheter), only significant differences were found among the different etiologies of renal failure, for the three survival estimates. A worse survival was observed for other etiologies and diabetics than for vascular (nephroangiosclerosis) or idiopathic etiology.
At 12 and 24 months, in diabetics, vascular, idiopathic and other etiology groups, respectively, the permeabilities were: PP: 55.1–29.0%, 76.4–65.7%, 72.2–59.6% and 41.7–15.6% (p=0.037 Log Rank-Mantel–Cox); APP:78.6–57.3%, 85.5–75.2%, 83.3–68.4% and 58.3–25.9% (p=0009), and SP: 82.2–58.9%, 89.1–76.8%, 83.3–68.8% and 66.7–31.7% (p=0.019, Fig. 2). However, the analysis of subcategories only showed worse primary permeabilities, for the grouping of diabetic etiologies and others vs vascular and idiopathic (p=0.01, p=0.062 and p=0.062). No significant differences were observed in assisted primary permeability or secondary permeability. The rest of the risk factors, such as the cases with central venous catheters, showed no significant differences (Table 2).
Estimates (%) of primary patency (PP), assisted primary patency(APP) and secondary (SP), at two years of follow-up, for the different prognostic factors, standard deviation (SD) and statistical significance of the differences between groups (P).
| PP | APP | SP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % | SD | p | % | SD | p | % | SD | p | |
| Age | |||||||||
| <70 years old | 51.5 | 0.065 | 0.875 | 67.7 | 0.064 | 0.865 | 69.7 | 0.062 | 0.929 |
| >Age70 | 50.0 | 0.070 | 60.7 | 0.068 | 62.7 | 0.067 | |||
| Sex | |||||||||
| H | 55.3 | 0.058 | 0.856 | 70.7 | 0.054 | 0.324 | 71.2 | 0.053 | 0.462 |
| M | 42.3 | 0.082 | 52.3 | 0.085 | 56.0 | 0.087 | |||
| Etiology | |||||||||
| DM | 29.0 | 0.081 | 0.037 | 57.3 | 0.091 | 0.009 | 58.9 | 0.089 | 0.019 |
| NAE | 65.7 | 0.067 | 75.2 | 0.060 | 76.8 | 0.059 | |||
| Idiopathic | 59.6 | 0.119 | 68.4 | 0.121 | 68.8 | 0.120 | |||
| Other | 15.6 | 0.131 | 25.9 | 0.144 | 31.7 | 0.164 | |||
| Dyslipidemia | |||||||||
| Do not | 52.2 | 0.059 | 0.771 | 63.1 | 0.058 | 0.246 | 65.9 | 0.058 | 0.238 |
| Yes | 48.9 | 0.081 | 66.5 | 0.077 | 66.9 | 0.076 | |||
| Cardiopathy | |||||||||
| Do not | 52.4 | 0.051 | 0.587 | 65.7 | 0.049 | 0.537 | 67.9 | 0.048 | 0.656 |
| Yes | 39.7 | 0.136 | 57.1 | 0.132 | 57.1 | 0.132 | |||
| Antiaggregant | |||||||||
| Do not | 53.8 | 0.053 | 0.240 | 67.9 | 0.051 | 0.705 | 69.1 | 0.050 | 0.882 |
| Yes | 37.4 | 0.109 | 50.3 | 0.110 | 54.0 | 0.112 | |||
| Previous CVC | |||||||||
| Do not | 50.1 | 0.051 | 0.784 | 65.6 | 0.049 | 0.745 | 67.7 | 0.049 | 0.582 |
| Yes | 56.3 | 0.135 | 56.3 | 0.135 | 56.3 | 0.135 | |||
| Side | |||||||||
| Straight | 49.1 | 0.079 | 0.592 | 63.6 | 0.075 | 0.640 | 63.6 | 0.075 | 0.362 |
| Left | 52.3 | 0.060 | 65.0 | 0.059 | 68.0 | 0.058 | |||
| Hypertension | |||||||||
| No | 57.7 | 0.110 | 0.505 | 60.1 | 0.113 | 0.999 | 60.6 | 0.113 | 0.841 |
| Yes | 49.3 | 0.053 | 65.4 | 0.051 | 67.7 | 0.050 | |||
This study demonstrates that surgical implantation of humerobasilic FAVn with superficialization and transposition in a single surgical act results in good patency rates with few complications during the follow-up period. If this intervention is performed in a single time, the basilic vein is dissected and mobilized, then the new path is created followed by the arteriovenous anastomosis. The main advantage of a single procedure is the greater speed in the cannulation of the vascular access and the shorter period of time of permanence of the central venous catheter, minimizing the risk of endovascular infections. The disadvantage lies in having as possible complications a greater number of mechanical problems related to the superficialization and transposition of a non-arterialized vein.
Some authors propose that the placement of this type of vascular access should be performed in a two steps procedure. First perform, the anastomosis between the basilic vein and the humeral artery, and 30–90 days later, perform superficialization and/or transposition when an adequate maturation is evidenced by echography.12 The publications describe three possible techniques for the superficialization of the basilic vein: (a) anterior transposition in the arm, by creating a new subcutaneous tunnel; (b) Anterior transposition in the arm by creating a lateral flap of skin and subcutaneous tissue and, (c) simple superficialization without transposition, although in this case it is difficult for the nursing staff to puncture.
Several studies have shown better survival curves with suprficialization of the basilic vein versus the implantation of prosthetic fistulas (pAVF).13,14 Among the complications associated with grafts, it should be mentioned the neointimal hyperplasia of the vein, mainly at the level of the venous anastomosis, with thrombosis and infection. According to some studies, up to 90% of the patients who presented thrombosis of their pAVF have hyperplasia of the venous neointima that cuses significant stenosis.15 This is important when deciding which type of vascular access to implant, especially in older patients with associated comorbidities.
A recent meta-analysis based on 8 studies including 859 HB S-T nAVF (366 single-procedure and 493 two surgical acts) concludes that there are no significant differences in terms of maturation rates, postoperative complications and permeabilities (PP, APP and SP).16 Studies reporting surgical complications in one act vs two surgical acts show adequate maturation (90–20% vs 95–18%), postoperative hematomas (17–4% vs 25–3%) and infections (18-0% vs 15-0%). As compared with other series we have a low rate of immediate postoperative complications (bruising, reoperation due to bleeding, infections or immediate thrombosis), results that support the safety of a single surgical act.
As far as permeability, there is a tendency in favor of the two vs one surgical acts, although without reaching statistical significance.16Table 3 compares the results from some of the main studies. In our series, permeabilities obtained (PP and SP) were similar to those reported in a single surgical act but lower than in the two acts. It should be noted that, according to the results obtained in a recent meta-analysis,20 diabetes mellitus predicts poor survival of the AVF. In this sense, our study has shown that patient's pathology is the only predictive variable of permeability (primary, primary and secondary) during follow-up. Table 2 shows the permeabilities at 2 years of follow-up: given the high number of censored patients and events during follow-up, we consider the two years an adequate time point to describe differences. And although the etiologies are varied (etiologies have been grouped with less than 5% representation in “other” etiologies, which are not analyzed in detail given their heterogeneity), it was found a different distribution of all permeabilities according depending on the etiology.
Comparative analysis of the permeabilities (primary and secondary) obtained in the main studies (1 and 2 surgical acts).
| Studies | Number procedures (subtype) | Primary P. | Secondary P. | ||
|---|---|---|---|---|---|
| 1 year | 2 years | 1 year | 2 years | ||
| Ozcan et al.17 | 106 | ||||
| 1 step: 47 | 70% | 64% | 76% | 72% | |
| 2 steps: 59 | 84% | 73% | 90% | 82% | |
| Vrakas et al.18 | 149 | ||||
| 1 step: 65 | 71% | 53% | 79% | 57% | |
| 2 steps: 84 | 87% | 75% | 95% | 77% | |
| Agarwal et al.19 | 144 | ||||
| 1 step: 61 | 82% | 81% | 91% | 80% | |
| 2 steps: 83 | 67% | 27% | 81% | 61% | |
| Fontseré et al. | 119 | ||||
| 1 step: 119 | 66% | 52% | 84% | 67% | |
According to our results diabetic patients and other etiologies presented worse permeabilities at 12 and 24 months than patients with vascular pathology or of unknown etiology (idiopathic). However, the analysis of subcategories only showed worse primary permeabilities, but not primary or secondary, in diabetic and other etiologies. This is probably due to the small number of patients, the high number of losses and events during the follow-up and the heterogeneity of the group of other etiologies (only present in 10% of cases). The reasons why the diabetic patients show worse permeability than vascular and idiopathic pathologies are not completely clear, but it is worth mentioning a greater platelet aggregation and endothelial damage, favoring the thrombosis and loss of vascular access.21 On the other hand, it is well known that atherosclerosis is more prevalent in diabetic patients, with more problems of arteries supplying adequate blood flow.22,23
Thus, it is recommended the implementation of an active program of monitoring and early detection of significant vascular access dysfunction based on first and second generation methods (vascular flow measurement).7 The differences obtained within our series could be explained on the basis that most patients from other areas of Catalonia were monitored only by first generation methods. Also, it is not always know the prevalence of diabetic patients in other published studies, a factor that, as we have shown, may have a negative impact on the survival of this type of native vascular access. Finally, unlike other surgical groups, we prioritize the implantation of this type of access even with limited veins available before the creation of a humerus-axillary prosthesis, a reason that may also go against our results. Among the main limitations of our study are that it is not prospective study and lacks a control group with superficialization and transposition of the basilic vein in two surgical steps.
ConclusionsAccording to the results obtained in the present study, the surgical implantation of S-T HB nAVF in a single surgical act offers good patency rates and a low number of complications. Diabetes mellitus is a factor that predict a worse surgical prognosis based on lower rates of permeability. Among the greatest advantages of this technique are a better optimization of social healthcare resources and a shorter duration of the central venous catheter.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fontseré N, Mestres G, Yugueros X, Jiménez M, Burrel M, Gómez F, et al. Fístulas arteriovenosas nativas humerobasílicas con superficialización y trasposición en un solo acto quirúrgico. Revisión de cinco años de experiencia. Nefrología. 2019;39:388–394.