Acute kidney injury (AKI) is associated with higher mortality and length of stay (LOS) for hospitalized patients. To improve outcomes, an electronic detection system could be a useful tool for early diagnosis.
MethodsA fully automated real-time system for detecting decreased glomerular filtration rate in adult patients was developed in our hospital, DETECT-H project. AKI was established according to KDIGO guidelines.
ResultsIn six months, 1241 alerts from 11,022 admissions were issued. Overall incidence of AKI was 7.7%. Highest AKI stage reached was: stage 1 (49.8%), 2 (24.5%) and 3 (25.8%), in-hospital mortality was 10.9%, 22.7%, 33.9% respectively and 57.1% in AKI requiring dialysis; mortality in stable CKD was 4.3%. Median LOS was 8 days versus 5 days for all patients. AKI was associated with a mortality of 3.18 (95% CI 1.80–5.59) and a LOS 1.52 (1.11–2.08) times as high as that for admissions without AKI. Multivariate analysis indicated that a LOS higher than 8 days was associated with AKI. Previous CKD was noted in 31.9% and AKI in 45.3% at discharge. As compared to the use of the detect system, only one third of CKD patients and half of AKI episodes were identified.
ConclusionsCKD and in-hospital AKI are under-recognized entities. Mortality and LOS are increased in-hospital patients with renal dysfunction. AKI severity was associated with higher mortality and LOS. An automated electronic detection system for identifying renal dysfunction would be a useful tool to improve renal outcomes.
El fracaso renal agudo (FRA) aumenta la mortalidad y la estancia hospitalarias (EH). El empleo de sistemas de detección electrónica podría ser una herramienta beneficiosa para mejorar estos resultados.
MétodosSe desarrolló un sistema de detección automático a tiempo real de pacientes ingresados con función renal alterada, denominado proyecto DETECT-H. El FRA se estableció de acuerdo con las guías KDIGO.
ResultadosEn 6 meses, 1.241 alertas fueron recogidas de 11.022 ingresos. La incidencia global del FRA fue del 7,7%. La distribución en función del estadio máximo del FRA alcanzado fue: estadio 1: 49,8%, estadio 2: 24,5% y estadio 3: 25,8%; con una mortalidad hospitalaria del 10,9, 22,7 y 33,9%, respectivamente. En el caso del FRA con necesidad de diálisis fue del 57,1%. La mortalidad en pacientes con enfermedad renal crónica (ERC) estable fue del 4,3%. La mediana de EH en pacientes detectados fue 8 vs. 5 días para todos los pacientes hospitalizados. El FRA se asoció con una mortalidad 3,18 (1,8-5,59) y una EH 1,52 (1,11-2,08) veces superior que aquellos ingresos sin FRA. El análisis multivariante indicó que el FRA se asociaba con la EH>8 días.
En los informes de alta, la presencia de ERC previa solo fue registrada en el 31,9% de los pacientes con ERC y el FRA hospitalario en el 45,3%.
ConclusionesLa ERC y el FRA intrahospitalario son entidades infradiagnosticadas. La mortalidad y la EH están aumentadas en pacientes con disfunción renal. La gravedad del FRA se asoció con mayor mortalidad y EH. Un sistema de detección automático para identificarlos podría ser útil para mejorar estos resultados.
Acute kidney injury (AKI) is a common, serious and expensive health problem and is an increasingly encountered complication among hospitalized patients.1,2
AKI management is complicated in part due to delay in detection and late nephrology referral.3–5 In the United Kingdom (UK), a recent report by the National Confidential Enquiry into Patient Outcomes and Death (NCEPOD)6 found that 30% of AKI cases occurring during hospitalization admission were avoidable, and that only 50% of patients with AKI received an overall standard of care that was considered good.3
In the past, measurement of the incidence and prevalence of AKI and analysis of outcomes have been hampered by the lack of an agreed definition.7 Most recently, Kidney Disease Improving Global Outcomes (KDIGO) criteria have provided consensus on a definition of AKI.8 This classification has allowed comparison between AKI studies, providing more evidence on incidence, the association with increased mortality and suggesting worse outcomes with increasing AKI stage.7 The KDIGO guideline for AKI is based on serum creatinine (sCr) and urine output criteria to define AKI and its stages of severity. KDIGO criteria have been validated9,10 and stages allow to predict patients’ outcomes as mortality, length of stay (LOS) and progression to chronic kidney disease (CKD).11,12 This has enabled healthcare professionals to start to attribute the causality of AKI and suggest optimal management and prevention strategies.13
Nevertheless, AKI is still an under diagnosed entity, with sub-optimal management and a late referral to nephrology services,6 which increase hospital costs considerably. Kerr et al. demonstrated that AKI prevalence in inpatients may be considerably higher than previously thought, more than 14%, and up to four fifths of cases may not be captured in routine hospital documentation.3 They estimate that the annual number of excess inpatients deaths associated with AKI in England may be above 40,000 and the annual cost of AKI-related inpatient care at 1.02 billion pounds (just over 1% of the National Health Service (NHS) budget).3
Recent real-time electronic alert systems that are based on changes in sCr can help identify cases of CKD and AKI. It is hoped that such early detection would lead to improved management and timely referral to a renal service.1 Furthermore, such early detection might reduce in-hospital mortality, AKI requiring renal replacement therapy (RRT), morbidity and health costs.14,15
To improve detection rates at our hospital, we developed a fully automated, daily electronic system which identifies all cases of renal dysfunction (CKD or AKI) according actual KDIGO criteria for inpatients over 14 years. The aim was to analyze clinical outcomes of patients with renal dysfunction and to validate the use of such detection systems.
Materials and methodsThe Complejo Hospitalario Universitario de Cáceres (CHUCC) provides services for 196,363 residents and specialist service to 409,537 from neighboring Health areas in Extremadura, with 520 inpatients beds in two hospitals situated 1.5km apart. There are ∼25,000 admissions each year excluding patients under 14 years. The hospital provides all main specialties except cardiac surgery and is a major regional hematology center. Many of the “acute” specialties are based at one campus (San Pedro de Alcántara Hospital), whereas geriatrics, ophthalmology, dermatology and plastic surgery are at the other (Nuestra Señora de la Montaña Hospital). The intensive care unit is at the first hospital.
We conducted a single-center, observational, retrospective study of all consecutive admissions of adults patients (aged >14 years) with decreased eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation detected between January to June 2014 at CHUCC.
The NHS in Extremadura has a computerized medical record with several information systems interconnected. JARA database which includes all inpatient and outpatient health activity and CORNALVO database which collect the laboratory results.
The nephrology department in our hospital has launched an initiative called “Detection of renal dysfunction in hospitalized patients” (DETECT-H project). DETECT-H is an electronic nephrology tool to detect inpatients with reduced estimated glomerular filtration rate (eGFR) in other services, out of nephrology department. DETECT-H software is based on the integration of JARA and CORNALVO databases. The two databases include in-hospital and ambulatory sCr values of a single patient linked with each other, therefore a longitudinal follow-up of creatinine-based renal function is possible. The software was tested in pilot form between November and December 2013. After checking the reliability of renal dysfunction detection in in-hospital patients, we analyzed data from the first six months in 2014, when the program had been fully implemented, and the project is still running.
A daily systematic message was sent to Nephrology department via email when any inpatient have an eGFR <60mL/min/1.73m2 during the admission.
Messages contain a personal identification code (PIC), the room number and the admission service. Patients detected were included in the study. Exclusion criteria were patients on RRT or age <14 years.
The detection messaging system was set-up within the DETECT-H software, used by the two hospitals in CHUCC. The message was only sent to the nephrologist and was not used to guide management. As a preliminary study, messages were not sent to clinicians and patients were managed in the usual way by their medical team. Patient referral to Nephrology department was entirely at discretion of their hospital clinician.
All sCr measurements were retrospectively recorded since the admission date and during the hospitalization period to analyze renal function along admission.
Additionally, previous sCr measurements until six months before admission were collected. The lowest sCr value between 6 months prior to date of admission was considered the “baseline” sCr. When a previous sCr was not able, the lowest sCr during admission was considered as “baseline” sCr. CKD diagnosis was established and classified according to sCr KDIGO criteria.16 Patients with previous reduced eGFR were considered as CKD patients if a threshold of eGFR <60mL/min/1.73m2 was present three months before the admission or longer. Stable CKD was considered in patients with previous reduced eGFR who do not achieved AKI criteria.
AKI diagnosis was established and classified according to sCr KDIGO criteria and AKI stage was defined by the highest sCr within the episode. With our software, one patient admission could result only in a single detection, and the highest serum creatinine value reached during the admission was used to stage AKI.
The Jaffe method is used for sCr measurement. Patient characteristics on the detection were collected from electronic medical record.
Analyses were performed in accordance with the Declaration of Helsinki and the guidelines of the institutional review board of the CHUCC. The study was approved by the CHUCC Ethics Committee.
OutcomesThe main study outcome measure was the prevalence of renal dysfunction in hospitalized patients, classified as AKI or CKD according to KDIGO definition, using an electronic automated detection system. We were also interested in learning how many of these patients had their CKD or AKI documented and recognized in the medical record. Furthermore, we evaluated the distribution of patients with renal dysfunction by service specialty, LOS and mortality in detected patients. Also we collected previous CKD record (CKDr) and in-hospital AKI diagnosis (AKId) reported on electronic medical records.
Statistical analysisStatistical performed using IBM SPSS Statistics (SPSS, Chicago, IL, USA). Categorical variables were expressed as percentages and continuous variables were expressed as mean and interquartile range (IQR). Differences in LOS and mortality between groups were evaluated using the two-sided χ2 test for categorical variables. Variables statistically significant in univariate analyses, age and gender, were included in a multivariate analyses using logistic regression model with enter method. A p-value <0.05 was considered statistically significant.
ResultsThere were 11,022 hospital admissions in 6 months, and the number of detection messages issued were 1241 (11.3% of the admissions), related to 1079 different patients. There were 162 repeated messages corresponding with 13.1% of readmission patients; these patients triggered two or more messages. Median age was 77 years (first and third quartiles 70, 81), 53.9% were males.
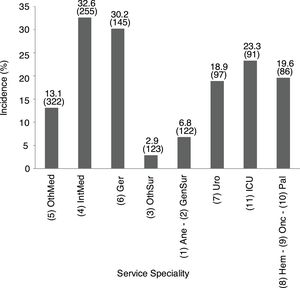
Incidence of patients with impaired renal function by service specialityThe specialty incidence of patients with renal dysfunction detected is shown in Fig. 1.
Incidence of patients with renal dysfunction by service speciality. Data show incidence and number of patients. 1Ane, anesthesia; 2GenSur, general surgery; 3OthSur, other surgery specialties; 4IntMed, internal medicine; 5OthMed, other medicine specialties; 6Ger, geriatrics; 7Uro, urology; 8Hem, hematology; 9Onc, oncology; 10Pal, palliative medicine; 11ICU, intensive care unit.
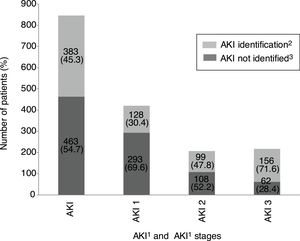
Overall incidence of AKI, using KDIGO criteria, for inpatients (defined as an admission including overnight stay) was 7.7% of total admissions. Among 1241 messages generated, AKI criteria were present in 846 (68.2%) related to 728 patients. AKI criteria were achieved in 3.6% of in-hospital patients with previous preserved eGFR, while were present in 53.2% of patients with previous CKD. Percentages of detection messages by AKI stage were analyzed per admission episode. The proportion of admission episodes generating an AKI message with highest stages 1, 2 and 3, respectively was 421 (49.8%), 207 (24.5%), 218 (25.8%). In terms of caregiver recognition, the diagnosis of AKI as a major discharge diagnosis was documented at discharge in 33.2% of patients who met AKI criteria, and 45.3% AKI episodes were recognized during the hospitalization with some documentation; in stage 1: 30.4%, stage 2: 47.8% and stage 3: 71.6%. AKI was recognized more often by the physician when the stage was higher (Fig. 2).
AKI identification at discharge according to KDIGO stages. 1AKI, acute kidney injury. 2AKI identification: percentage of AKI episode present and detected by DETECT-H software and documented by clinicians. 3AKI not identified: percentage of AKI episode present and detected by DETECT-H software but not documented by clinicians.
Renal function before hospitalization was known in 1042 detected patients (84%). Patients with a baseline eGFR >60mL/min/1.73m2 were 47.3%. Previous CKD distribution was stage 3a: 25%, stage 3b: 17.7%, stage 4: 9.1%, and stage 5: 0.9%. Similar to the findings with the AKI notification, pre-existing CKD was recognized more often when renal dysfunction was worse. Only 31.9% of patients with CKD stage 3a or higher had this antecedent data reflected in their medical record. The rates were higher with worsening stages of CKD; stage 3a: 9.1%, stage 3b: 35.2%, stage 4: 54.1%, stage 5: 57.7%. During the study period, 7.5% of stable CKD patients had an AKI diagnosis during their hospital admission.
Renal function in detected patientsTable 1 summarizes the renal function stages according to KDIGO classification in the studied population (Table 1). In 172 patients (13.9%) only one sCr measurement was available while admission.
Renal function according to eGFR1.
| eGFR1 before admissionN=1042 (84%) | eGFR1 at admissionN=1241 (100%) | eGFR1 at dischargeN=1069 (86.1%) | |
|---|---|---|---|
| eGFR1 >60mL/min/1.73m2 (%) | 47.3 | 11.6 | 29.6 |
| eGFR1 59–45mL/min/1.73m2 (%) | 25.0 | 28.2 | 22.9 |
| eGFR1 44–30mL/min/1.73m2 (%) | 17.7 | 23.1 | 23.5 |
| eGFR1 29–15mL/min/1.73m2 (%) | 9.1 | 29.3 | 19.3 |
| eGFR1 <15mL/min/1.73m2 (%) | 0.9 | 7.8 | 4.7 |
1eGFR, estimated glomerular filtration rate.
Median LOS for inpatients in CHUCC during the studied period was 5 days. Median LOS for patients prompting a detection message was 8 days (IQR first and third quartiles 4–13 days). Median LOS across all stages of AKI increased as the stage of AKI increased: 6 days for patients with stable CKD during the hospitalization episode, 8 days for AKI stages 1–2, 10 days for AKI stage 3 and 12 days for stage 3 requiring RRT, respectively (IQR first and third quartiles 3–10, 5–13, 6–14, 5–19 and 6–20 days, respectively). The LOS was statistically significant related to the evolution of sCr at discharge compared to the previous (p=0.001) with a direct linear relationship (Spearman's correlation coefficient of 0.145), and retained statistical significance in multivariate analysis (p=0.044). Multivariate analysis also indicated that a LOS higher than 8 days was associated with AKI. Table 2a shows the percentages of detections in different groups and the multivariate regression analysis for LOS higher than 8 days (Table 2a). AKI was associated with a longer LOS 1.52 (1.11–2.08) times as high as those for patients without AKI (Table 2a).
Length of stay in different groups.
| LOS17 >8 days, n (%) | P value – univariate analysis | P value – multivariate analysis | OR (95% CI) | |
|---|---|---|---|---|
| Sex | ||||
| Male; n=668 | 275 (41.2) | 0.088 | ||
| Female; n=572 | 263 (46.0) | |||
| Age (years) | ||||
| ≤80; n=821 | 343 (41.8) | 0.110 | ||
| ≥80; n=419 | 195 (46.5) | |||
| Ane1-GenSur2 | ||||
| Yes; n=121 | 70 (59) | 0.001 | 0.014 | 1.82 (1.13–2.92) |
| No; n=1119 | 468 (41.8) | |||
| OthSur3 | ||||
| Yes; n=123 | 54 (43.9) | 0.903 | ||
| No; n=1117 | 484 (43.3) | |||
| IntMed4 | ||||
| Yes; n=225 | 118 (46.3) | 0.297 | ||
| No; n=985 | 420 (42.6) | |||
| OthMed5 | ||||
| Yes; n=332 | 119 (37.0) | 0.007 | 0.035 | 0.71 (0.52–0.98) |
| No; n=918 | 419 (45.6) | |||
| Ger6 | ||||
| Yes; n=145 | 71 (49.0) | 0.149 | ||
| No; n=1095 | 467 (42.6) | |||
| Uro7 | ||||
| Yes; n=97 | 14 (14.4) | <0.001 | <0.001 | 0.20 (0.11–0.37) |
| No; n=1143 | 524 (45.8) | |||
| Hem8-Onc9-Pal10 | ||||
| Yes; n=86 | 46 (53.5) | 0.050 | ||
| No; n=1154 | 492 (42.6) | |||
| ICU11 | ||||
| Yes; n=91 | 46 (50.5) | 0.152 | ||
| No; n=1149 | 492 (42.8) | |||
| Exitus | ||||
| Yes; n=185 | 75 (40.5) | 0.397 | ||
| No; n=1055 | 463 (43.9) | |||
| CKDr12 | ||||
| Yes; n=396 | 184 (46.5) | 0.134 | ||
| No; n=844 | 354 (41.9) | |||
| eGFR13before admission (mL/min/1.73m2) | ||||
| Yes; n=493 | 219 (44.4) | 0.641 | ||
| No; n=549 | 236 (43.0) | |||
| eGFR13at admission (mL/min/1.73m2) | ||||
| Yes; n=144 | 74 (51.4) | 0.039 | ||
| No; n=1096 | 464 (42.3) | |||
| eGFR13at discharge (mL/min/1.73m2) | ||||
| Yes; n=317 | 170 (53.6) | 0.014 | 0.044 | 0.75 (0.56–0.99) |
| No; n=751 | 341 (45.4) | |||
| AKId14 | ||||
| Yes; n=412 | 205 (49.8) | 0.001 | ||
| No; n=828 | 333 (40.2) | |||
| AKI15stage 1–3 | ||||
| Yes; n=846 | 412 (48.7) | <0.001 | 0.009 | 1.52 (1.11–2.08) |
| No; n=373 | 121 (32.4) | |||
| Need RRT16 | ||||
| Yes; n=21 | 10 (47.6) | 0.690 | ||
| No; n=1219 | 528 (43.3) | |||
1Ane, anesthesia; 2GenSur, general surgery; 3OthSur, other surgery specialties; 4IntMed, internal medicine; 5OthMed, other medicine specialties; 6Ger, geriatrics; 7Uro, urology; 8Hem, hematology; 9Onc, oncology; 10Pal, palliative medicine; 11ICU, intensive care unit; 12CKDr, chronic kidney disease record; 13eGFR, estimated glomerular filtration rate; 14AKId, acute kidney injury diagnosis; 15AKI stages 1–3, acute kidney injury stages 1–3; 16RRT, renal replacement therapy; 17LOS, length of stay.
In-hospital mortality for all patients prompting a detection message was 14.9% (185 patients) as opposed to 3.7% for overall in-hospital patients in the same period. Mortality increased with greater severity of renal dysfunction: 4.3% in CKD stable patients, and 10.9%, 22.7%, 33.9% in AKI stage 1, 2 and 3 respectively, reaching 57.1% in patients with AKI stage 3 who required RRT (continuous or intermittent). In-hospital mortality increased as eGFR declined. Mortality according renal function before admission in detected patients by DETECT-H system was: CKD stage 1 26.0%, 2 15.8%, 3a 9.2%, 3b 20.1%, 4 15.8%, 5 33.3%.
Percentages of mortality in different groups and multivariate statistical analysis are shown in Table 2b. Multivariate regression analysis indicated that AKI was associated with mortality rates 3.18 (95% CI 1.80–5.59) times as high as that for admissions without AKI and, AKI requiring RRT with a 5.36 (95% CI 2.02–14.26) times as high as for patients without RRT (Table 2b).
Mortality in different groups.
| Mortality, n (%) | P value – univariate analysis | P value – multivariate analysis | OR (95% CI) | |
|---|---|---|---|---|
| Sex | ||||
| Male; n=668 | 105 (15.7) | 0.424 | ||
| Female; n=572 | 80 (14.0) | |||
| Age (years) | ||||
| ≤80; n=821 | 93 (11.3) | <0.001 | 0.003 | 1.81 (1.22–2.68) |
| ≥80; n=419 | 92 (21.9) | |||
| Ane1-GenSur2 | ||||
| Yes; n=121 | 15 (12.4) | 0.502 | ||
| No; n=1119 | 170 (15.2) | |||
| OthSur3 | ||||
| Yes; n=123 | 5 (4.1) | <0.001 | 0.144 | 0.45 (0.15–1.32) |
| No; n=1117 | 180 (16.1) | |||
| IntMed4 | ||||
| Yes; n=225 | 50 (19.5) | 0.023 | 0.405 | 1.34 (0.67–2.68) |
| No; n=985 | 135 (13.7) | |||
| OthMed5 | ||||
| Yes; n=332 | 24 (7.5) | <0.001 | 0.209 | 0.63 (0.31–1.29) |
| No; n=918 | 161 (17.5) | |||
| Ger6 | ||||
| Yes; n=145 | 33 (22.8) | 0.009 | 0.517 | 1.29 (0.60–2.81) |
| No; n=1095 | 152 (13.9) | |||
| Uro7 | ||||
| Yes; n=97 | 1 (1.0) | <0.001 | 0.014 | 0.08 (0.01–0.59) |
| No; n=1143 | 184 (16.1) | |||
| Hem8-Onc9-Pal10 | ||||
| Yes; n=86 | 30 (34.9) | <0.001 | 0.002 | 3.25 (1.55–6.80) |
| No; n=1154 | 155 (13.4) | |||
| ICU11 | ||||
| Yes; n=91 | 27 (29.7) | <0.001 | 0.059 | 2.07 (0.97–4.40) |
| No; n=1149 | 158 (13.7) | |||
| CKDr12 | ||||
| Yes; n=396 | 69 (17.4) | 0.104 | ||
| No; n=844 | 116 (13.7) | |||
| eGFR13before admission (mL/min/1.73m2) | ||||
| Yes; n=493 | 88 (17.8) | 0.150 | ||
| No; n=549 | 79 (14.4) | |||
| eGFR13at admission (mL/min/1.73m2) | ||||
| Yes; n=144 | 26 (18.1) | 0.263 | ||
| No; n=1096 | 159 (14.5) | |||
| AKId14 | ||||
| Yes; n=412 | 93 (22.6) | <0.001 | 0.664 | 1.09 (0.74–1.60) |
| No; n=828 | 92 (11.1) | |||
| AKI15stage 1–3 | ||||
| Yes; n=846 | 167 (19.7) | <0.001 | <0.001 | 3.18 (1.80–5.59) |
| No; n=373 | 16 (4.3) | |||
| Need RRT16 | ||||
| Yes; n=21 | 12 (57.1) | <0.001 | 0.001 | 5.36 (2.02–14.26) |
| No; n=1219 | 173 (14.2) | |||
1Ane, anesthesia; 2GenSur, general surgery; 3OthSur, other surgery specialties; 4IntMed, Internal medicine; 5OthMed, other medicine specialties; 6Ger, geriatrics; 7Uro, urology; 8Hem, hematology; 9Onc, oncology; 10Pal, paliative medicine; 11ICU, intensive care unit; 12CKDr, chronic kidney disease record; 13eGFR, estimated glomerular filtration rate; 14AKId, acute kidney injury diagnosis; 15AKI stages 1–3, acute kidney injury stages 1–3; 16RRT, renal replacement therapy.
Our primary goal was to develop a clinical tool which was able to detect renal dysfunction in hospitalized patients based on a fully automated real-time electronic detection system. Our hypothesis was that many cases of CKD and AKI went unrecognized and could benefit from nephrology consultation.
We collected outcome data for six months after the introduction of the DETECT-H project with more than a thousand detection messages generated. The methodology is transferable to other hospitals.
A multidisciplinary collaborative network joined to the introduction of electronic medical records and interconnected databases in hospitals increases the possibility of using software to optimize the automatic detection of biochemical alterations in acute and chronic kidney disease.17,18 Our software allows an automatic identification of patients with low eGFR and offers the promise of using this identification to improve outcomes.
These data shows that in our population, 11.3% of hospital admissions involved patients with renal dysfunction and 7.7% reached AKI criteria, similar to rates described in other studies.1,3 Inpatient mortality increased with worsening levels of renal dysfunction. Stable CKD and AKI stages were independently associated with mortality in a graded fashion. LOS for the hospital admission also increased as renal dysfunction worsened, being significantly higher with higher AKI stages but also in stable CKD patients.
Porter et al. reported a hospital-wide fully automated electronic system for AKI detection1,2 based on Risk, Injury, Failure, Loss, End-stage kidney disease (RIFLE) and Acute Kidney Injury Network (AKIN) criteria that confirm the high incidence of AKI in-hospital patients. AKI and CKD are related to each other and involve a significant morbidity,19–21 thus, in our study, we expanded our detection system to include all levels of renal dysfunction, not only AKI, in a large teaching hospital. We feel that this broader inclusion criteria is important to allow the best possible care for all patients with renal dysfunction (i.e. CKD and AKI patients).
The most sobering finding in our study is that both AKI and CKD are often not documented in the medical record despite being present. The prevalence of this failure of recognition was as high as 50% in some instances and generally decreased as the severity of renal dysfunction increased. While we cannot definitively state that this absence of documentation negatively impacted care, we cannot help but hypothesize that the early recognition of AKI or CKD may have altered care and led to improved outcomes. Future studies to use the alert to notify clinicians of the presence of AKI or CKD will have to be done in order to assess the impact on care and outcomes.
The DETECT-H system as used in this study was utilized in a retrospective fashion to assess baseline recognition of AKI and CKD by clinicians. Thus, an advisory message was not sent to the clinician end-user, and thus no active interventions in clinical practice were performed. Wilson et al. reported in a single-blind, parallel group, randomized controlled trial no differences were found in the primary composite of relative maximum increase in creatinine, dialysis or death endpoint between randomized patients to receive an AKI alert or usual care.22 While this one study did not demonstrate benefit, our finding of the low rate of recognition of AKI and CKD in our hospital system may present an opportunity for care improvement.
In summary, our study highlights the poor awareness that the medical community (non-nephrologists) have in recognizing renal disease. Thus, greater education is required as well as a study regarding the use of e-alerts to improve care.
Financial disclosureThe authors declare no financial disclosure.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors gratefully acknowledge the financial grant support of Amgen Foundation for the interface software implementation between databases and the detection system platform.