End-Stage Renal Disease (ESRD) is one of the major causes of morbidity and mortality worldwide. Although the incidence of ESRD is relatively stable, the prevalence of maintenance dialysis is increasing, and it is expected to reach a staggering 5439 million patients worldwide by 2030. Despite the great technological evolution that has taken place in recent years, most patients are still treated with in-centre haemodialysis and their prognosis remains far from desirable.
Since 1980, there has been an increasing interest in the development of a portable device for renal replacement therapy (RRT), which ultimately led to the creation of the Wearable Artificial Kidney (WAK) and the Wearable Ultrafiltration (WUF) system. Portable RRT devices may be acceptable alternatives that deal with several unmet clinical needs of ESRD patients. So far, 3 important human studies with WAK and WUF have been carried out and, although these devices require considerable technological improvement, their safety and efficacy in solute clearance and fluid removal is undeniable.
In this article, we review the evolution of the WAK and the WUF and the main clinical trials performed, highlighting some of their technical features. Some of the main possible clinical advantages that could be achieved with these devices, as well as some economic aspects, are also pointed out.
In the future, all renal replacement therapy techniques should evolve to perfectly match the clinical and personal needs of each patient, allowing for an improved health-related quality of life.
La enfermedad renal crónica terminal (ERCT) es una de las principales causas de morbimortalidad mundial. Aunque la incidencia de esta enfermedad es relativamente estable, la prevalencia en diálisis está aumentando, y se espera que llegue a la cifra de 5.439 millones de pacientes en todo el mundo en el año 2030. A pesar de la gran evolución tecnológica ocurrida en los últimos años, la mayoría de los pacientes continúan siendo tratados con hemodiálisis, y su pronóstico queda lejos de lo deseable.
Desde 1980, existe un interés creciente en el desarrollo de dispositivos portátiles para la terapia de sustitución de la función renal (TSFR), y que llevaron a la creación del Wearable Artificial Kidney (WAK) y del Wearable Ultrafiltration (WUF) system. Estos pueden ser alternativas aceptables que permiten alcanzar las necesidades de los pacientes con ERCT, que hasta ahora no se han alcanzado. A pesar de que estos dispositivos necesitan mejoras tecnológicas, su seguridad y eficacia en el aclaramiento de solutos y la eliminación de fluidos es innegable.
Revisamos la evolución del WAK y del WUF, y los principales ensayos clínicos desarrollados, destacando algunas de sus particularidades tecnológicas. Adicionalmente, señalamos algunas de las posibles ventajas clínicas que podrían ser alcanzadas con estos dispositivos, así como algunos aspectos económicos.
En el futuro, todas las TSFR deben evolucionar para satisfacer todas las necesidades clínicas y personales de cada paciente, permitiendo una mejor calidad de vida relacionada con la salud.
According to the 2010 Global Burden of Disease report, Chronic Kidney Disease (CKD) is the 18th most common cause of death.1 Although the incidence of End Stage Renal Disease (ESRD) has relatively remained stable, the prevalence of maintenance dialysis has almost doubled from 1990 to 2010, increasing from 165 per million population (pmp) to 284 pmp2 and is expected to rise even further in the coming decade.3 By 2030, it is predicted that the number of patients receiving Renal Replacement Therapy (RRT) is expected to reach 5439 million worldwide.3 Although there exists considerable variability in the choice of appropriate RRT modality all over the world, most patients are treated with in-centre hemodialysis (HD), which in most countries exceeds 90% of the incident and 60% of the prevalent ESRD population.4 Despite numerous advances in the field of RRT, outcomes related to the quality of life and morbi-mortality in ESRD patients have not reached the expected levels. A growing body of evidence seems to indicate that more prolonged and more frequent dialysis therapies are associated with improved outcomes,5–7 which is in full agreement with both hemodynamic and renal physiology. However, most HD therapies are still exclusively performed intermittently using large immovable machines, installed in hemodialysis centers. It is also known that such treatments severely restrict the activities of daily living (ADL) of most ESRD patients. Due to this phenomenon, there has been an increasing interest in the development of a fully portable HD device since the early 1980s. With the advent of nanotechnology and miniaturization the first truly portable device meant for RRT, i.e. the Wearable Artificial Kidney (WAK), was first realised in 2005.8
ESRD and the clinical rationale for WAKCardiovascular disease (CVD) is the most common cause of death in dialysis patients. In addition to traditional risk factors, CVD morbi-mortality is also associated with uremia-related factors (URF) such as fluid overload, hyperphosphatemia, anemia, left ventricular hypertrophy, chronic inflammation and endothelial dysfunction.9–12 Due to the inherent inability of the traditional thrice weekly HD regimen to completely replace renal function and to optimize all URF, ESRD patients are required to follow stringent dietary restrictions (especially in potassium, phosphorus and total fluid intake) and to regularly consume a large number of oral medications. Recent studies have suggested that some of the benefits of high frequency and long duration dialysis are similar to those associated with kidney transplantation, due to the achievement of greater solute clearance, better volume control, better nutritional status and a higher health-related quality of life (HR-QOL).13–15 It is also accompanied by a reduction of ESRD related complications such as anemia, hypertension, hospitalization and need for additional medication (e.g. such as phosphate binders and antihypertensive therapy).16 Improvement of the blood pressure control associated with a reduced risk of intradialytic hypotension and a more physiological ultrafiltration (UF) rate during prolonged HD, may prevent cardiac stunning and thus reduce the risk of CVD related morbi-mortality in ESRD patients.
Residual Renal Function (RRF) plays a key role in ESRD patients especially for fluid, salt and phosphorus excretion, for clearance of middle size molecules and also for endogenous vitamin D and erythropoietin production.17,18 It is associated with better patient survival and greater health-related quality of life (HR-QOL).19 Generally, conventional HD treatments are performed with a fixed dose of thrice-weekly, without considering RRF. Preservation of RRF requires a holistic approach through regular monitoring, blood pressure control optimization, elimination of nephrotoxins and an individualized dialysis prescription.
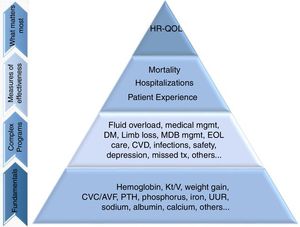
Most dialysis patients have low HR-QOL which is an independent predictor of mortality in this population.20–22 Dismal HR-QOL scores are partly explained by the co-existing comorbidities, but also by depression and the relatively high symptom burden.23,24 Conventional HD regimen not only markedly limits patient freedom, but also can be associated with severe post-dialysis fatigue that can further negatively impact HR-QOL.24,25 HR-QOL is a critical issue, being used to assess the effectiveness of healthcare interventions and it is becoming as important as morbidity and mortality while evaluating outcomes in dialysis patients.26 Manns et al.27 reported 30 important concerns for ESRD patients and found that although they do care about effects of dialysis modality on overall survival, they also worry about fatigue, depression, better quality of life, a good dialysis access, the ability to travel and to exercise and to eliminate barriers that hamper their ADL. Many ESRD patients have expressed interest in portable dialysis options.27,28 Since 2009, the US Institute of Medicine and the Patient Centred Outcomes Research Institute have promoted patient-centred care and it is also incentivized by the US Centres for Medicare and Medicaid services in designing quality programs for the ESRD population.29 Based on this concept, Nissenson et al. proposed a Maslow-like “quality pyramid” (Fig. 1), in which HR-QOL occupies the apex, supported by mortality, hospitalization and patient experience.30 According to this, dialysis patients’ treatment, which until recently was focused only on the assurance of fundamental clinical aspects (such as volume control, anemia and calcium-phosphorus metabolism) and outcomes, must also aspire for goals such as improved HR-QOL.
The patient-focused quality pyramid. “Fundamentals” are the basic clinical data, “Complex Programs” refers to clinical programs based on fundamental clinical areas; “Measures of effectiveness” refers to primary outcomes driven by lower complex programs and fundamental clinical areas of focus; “What matters most” are the outcomes that improve HR-QOL. AVF, arteriovenous fistula; CVD, cardiovascular disease; CVC, central venous catheter; EOL, end of life; HR-QOL, health-related quality of life; MBD, mineral and bone disorder; Med, medical; mgmt, management; Pt., patient; PTH, parathyroid hormone; tx, treatment; URR, urea reduction ratio.
Adapted from Ref. 31.
The initial idea of the WAK traces back to the 1970s. However, it was limited by the technologies available at that time.31–33 Mobility was one of the major limitations found, since the earlier device weighed almost 6.35kg (14 pounds).31 Only recently, the developments in technology and miniaturization have made wearable dialysis portable devices relatively practicable. The first trial to assess safety and efficacy of the WAK was reported in 2005 in animal models.34 Two years later, this device was tested in a pilot study involving 8 HD patients with a mean age of 51.7 years.35 They were connected to a device weighing 5kg (Xcorporeal Inc, Los Angeles, CA, USA), using a standard HD vascular access. The total system was composed of a bi-compartmental circuit, one for the blood and the other for the dialyzer; a polysulfone 0.6m2 high flux dialyser (Gambro Dialysatoren, Hechingen, Germany); a pulsatile blood pump, powered by a standard 9-V battery; four micro-pumps (Sorenson, West Jordan, UT, USA) used to infuse heparin into the blood circuit, to infuse sodium bicarbonate, magnesium, and calcium acetate into the dialysate circuit, and to control ultrafiltration. A series of sorbent canisters containing urease, activated charcoal, hydroxyl zirconium oxide and zirconium phosphate were used to regenerate dialysate. For safety two sensors for detection of air bubbles and of blood flow stoppage were used. The mean treatment time was 6.4 (SD±2.0) h. There was no evidence of important cardiovascular changes, hemolysis, serum electrolytes disturbances or acid-base balance disturbances. There was a statistically lower mean body weight after treatment. Clearance rates for urea and creatinine were much lower than those typically achieved in conventional HD. The mean blood flow was 58.6mL/min, with a dialysate flow of 47.1mL/min. The clotting of the vascular access that was observed in two patients was related to low heparin dose. In one patient a fistula needle got dislodged, but the blood pump stopped immediately, without any sequelae. The other technical problem occurred due to the accumulation of carbon dioxide bubbles in dialysate circuit, but this did not imply the discontinuation of the treatment. Despite the above technical difficulties, the patient's feedback was encouraging.
A growing body of evidence, involving New York Heart Association (NYHA) class III and IV congestive heart failure (CHF) patients resistant to diuretics, suggests that the removal of excess fluid, cytokines and a myocardial depressant factor through ultrafiltration therapy is associated with better outcomes.36 However, when UF is performed during a regular 4–6h HD session, it can result in hypotension and hemodynamic instability. Gura et al. in 2008, described the first wearable hemofiltration device (WUF) to manage fluid overload.37 This pilot study enrolled 6 fluid-overloaded HD patients in which isolated UF was applied for 6h. The average blood flow was 116ml/min and UF rate ranged between 120 and 288ml/h. Cardiovascular and biochemical parameters remained stable and there were no major complications. Clinically, the WUF can potentially reduce the incidence of acute pulmonary edema, ascites and other hypervolemic manifestations in patients with CHF NYHA Class III and IV.
In 2016, Gura et al published the first-ever human clinical trial which involved a 24h WAK treatment.38 Seven HD patients were selected to perform a WAK treatment for 24hours. Their mean age was 49 years and 3 had CHF. The mean blood flow was 42ml/min and dialysate flow was 43ml/min. Mean weighted-average concentrations of blood urea nitrogen (BUN) and β2-microglobulin were significantly lower during the 24-hour WAK treatment than those achieved in the previous 48-hour period by the conventional HD treatment. The mean UF volume of the 5 subjects that completed the 24-hour treatment was 1002ml and there were no significant hemodynamic changes. The treatment was stopped in 2 patients due to technical complications: namely clotting of the blood circuit in 1 subject and the appearance of a pink discoloration in the dialysate, without analytic evidence of hemolysis, in another. After the seventh patient, the clinical trial was discontinued due to technical issues, such as development of excessive carbon dioxide bubbles in the dialysate circuit, tubing kinks which caused fluctuation in blood and dialysate flow rates related to inconstant pump function. There were no important cardiovascular changes, acid–base disturbances, or electrolytic serum disturbances. The target UF was achieved and there was no need of dietary restriction or phosphorus-binding medications. Patients reported a significantly greater satisfaction with the WAK, owing to the greater freedom, its convenience and its flexibility.38
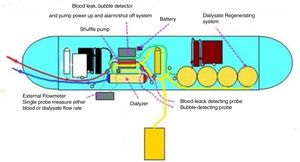
Technical aspects of WAK and WUFThe Wearable Artificial Kidney (WAK) is a wearable (using a belt) HD device that incorporates the basic components of a dialysis system into a wearable device, permitting miniaturization, patient-oriented management, and improved mobility.34 It works by drawing blood from the patient's vascular access and, using heparin and a pump system, and circulates it through the blood channel into the dialyzer. The dialyzed blood is then returned to the patient. The dialysate and the blood circulates in a counter-current direction. Another pump empties the spent dialysate into a collection bag. After going through a series of sorbents and being infused with a solution containing sodium bicarbonate, the dialysate is returned to the dialyser.
The mainly technical components are as follows: pumping systems; dialysis membranes; dialysis regenerations; patient monitoring systems and power sources (Fig. 2).
The WAK/WUF pumping system possibly is the most critical technical component since it is responsible for ensuring adequate blood flow, precise fluid exchange and drug infusion. The pumping system can be further subdivided into blood pumps and other fluid pumps. Blood pumps are responsible for extracting blood from the patient's vascular access, and for providing the minimum reliable and adjustable blood flow through the filter and for returning the filtered blood to the patient. Importantly, all this dynamic flow has to be done without hemolysis and with the highest possible biocompatibility. There are different types of blood pumps, such as peristaltic, shuttle, rotary and finger pumps with different specifications.39 Fluid pumps, on the other hand allow for the delivery of drugs, medications, anticoagulants, antibiotics etc. or remove fluids and solutes from the extracorporeal circuit. Additionally, there are at least two more types of fluids pumps: kinetic centrifugal and turbine pumps.39 At the moment there is no WAK pump that has incorporates all the best characteristics into a single integrated system.
WAK is a portable device used for long-term hemodialysis, so it is easily understood that in addition to the usual requirements of a dialysis membrane, the membranes used in this device should have some additional features related to portability and dialysis time. In fact, WAK dialysis membranes should have a geometry which allows for a replaceable disposable pump, an effective surface area and pore distribution for longer treatment time and cannot have any associated haemolysis.47 In the last years several advances in the development of dialysis membranes were made, namely the lightweight hemofilter unit33 and the application of mechanical vibration to induce high shear stress at the membrane surface with the aim of preserving membrane morphology and function for extended time periods.40 Other flourishing areas for dialysis membranes are the development of improved biocompatible materials and nanotechnology.
Another important feature of the WAK is a dialysate regeneration system that has to be lightweight, has to replace smart sorbent material with high adsorption capacity of uremic toxins and also has to accurately monitor the dialysate composition (with pH, temperature, volume, composition and bacterial contamination sensors). These requirements have been largely achieved with the use of sorbent technology.47 Kim et al demonstrated that in vitro, a cold dialysate regeneration system using a small volume of dialysate can have results comparable to conventional HD.41 A promising system for the application of the WAK is the REDY cartridge, which allows for the regeneration of 6L of dialysate per dialysis session. This cartridge is composed of charcoal, urease, cation and an anion exchanger. The evolution of this technology could overcome some of the previous problems related to the toxicity of aluminium and acidosis. However, this is yet to be miniaturized.34
Another key component of the WAK/WUF is the patient monitoring system. This is comprised of several sensors: fluid balance, pressure maintenance in the dialysis system, pump power battery, blood leakage and bubble detection, as well as vascular access disconnection.42 In the future, it is intended that the control of these systems should be done remotely in order to continuously monitor and adjust the clinical parameters of the treatment.
The power consumption of a WAK with a maximum pump flow rate of 120ml/min requires less than 5W,47 limiting the use of lithium batteries. A promising innovations for WAK/WUF could be thin-film, solid-state batteries42 and flexible batteries.43
The vascular access is the lifeline of most HD patients. The WAK/WUF vascular access must allow comfortable, prolonged and frequent dialysis treatments, without interference in ADL. AVF is the preferred vascular access for HD. However, a small needle dislodgment can result in severe complications, such as active bleeding, putting the patient's life at risk. Similar complications may also occur in grafts. Prolonged HD with these two portable devices will need a modified vascular access system with safe and convenient connection/disconnection systems, and with reduced risk of biofilms formation and coagulation. Taking previous experiences into consideration, it seems reasonable that the future WAK/WUF vascular access should be similar to a reduced lumen CVC with optimized aspects such as a port and more biocompatible materials which will have reduced associated risk of infection and coagulation.
WAK and WUF economical aspectsAlthough ESRD typically affects elderly people, many young people with active professional life are also affected by it. As a result, ESRD in young people leads to interruptions in schedules, reduced capacity to work due to forced absenteeism and sometimes even forces early retirement. Kaitelidou et al.44 in an economic evaluation of HD in Greece demonstrated an overall loss of 2046 years due to mortality and a potential productivity loss amounting to 9.9 million Euro, according to human capital approach (HCA). Importantly, in this study the total morbidity cost due to absence from work and early retirement was estimated at an amount exceeding 273 million Euro according to HCA.
In selected patients WUF treatment can possibly reduce morbi-mortality, by reducing the number of hospital admissions, the length of hospital stay, the ICU utilization and overall drug consumption.44 It is likely that this treatment could be beneficial to ESRD patients in terms of a reduced number of hospitalizations per year due to acute decompensated heart failure (ADHF). About a decade ago the UNLOAD trial, which was conducted to investigate if UF therapy was superior to IV diuretics in the treatment of ADHF or not, clearly proved that UF therapy significantly reduced the CHF hospitalization rates. At 90 days, the UF group versus the IV diuretics group had fewer patients rehospitalised for heart failure (HF) (16 of 89 [18%] vs. 28 of 87 [32%]; p=0.037), fewer HF rehospitalisations (0.22±0.54 vs. 0.46±0.76; p=0.022), and fewer rehospitalisation days (1.4±4.2 vs. 3.8±8.5; p=0.022) per patient.45 We are also aware that hospitalizations for ADHF carry a significant economic burden for any healthcare system. For example it was estimated that in an Italian hospital, the average yearly cost per person for hospitalization for heart failure (HHF) was €11,100, of which €4300 euro was for the index hospitalization (39%), €5900 for the subsequent hospitalizations (53%), and the remaining €900 for non-hospital charges (8%).46 From the two above studies, it is not unreasonable to assume that the use of the WUF device could have similar results, i.e. a 50% reduction in rehospitalisations and a 50% reduction in rehospitalisation days if not more. Considering the costs in an Italian hospital, this reduction could hence lead to calculated savings of approximately €5100 per patient if we assume a 75% reduction in non-index-hospitalization costs owing to a 50% reduction in the number of HHF and 50% reduction in the length of HHF. Even if we consider a conservative figure of 5–10 HHF every year, which is typical in a large Italian hospital treating about 100 HD patients, this is a net benefit of between €25,500 and €51,000. Promoting alternative technologies is a strategy that may lead to better cost-effectiveness and cost-utility for ESRD patients. Also the WUF might reduce the need for the performance of isolated UF sessions in a HD centre which can cost as much as €287.90 per session.
WAK and WUF present and future directionsWAK and WUF should evolve to match the clinical and personal needs of each patient, allowing for a better HR-QOL and minimal restrictions in ADL. These aims require considerable technological improvement in the currently used dialysis equipment, in terms of safety, biocompatibility and portability.32 Certainly, these developments in miniaturization will be the key step to the implementation of the WAK/WUF.
The first challenge in the implementation of the WAK/WUF is the development of an appropriate vascular access that allows a constant blood flow in the range of 100ml/min, which is adequate for a continuous dialysis therapy. Given the risk of dislodgment, significant haemorrhage and other technical complications, such as air embolism, infection, and clotting, a dual reduced lumen catheter could be a possible solution. This catheter must be constructed using more biocompatible materials and skin exit site technologies, which must ensure minimal risk of infection and clotting. Another important feature is the easy connection and disconnection systems that can be controlled by the patient himself. This catheter should help minimize the risk of associated stenosis.
As previously mentioned, antithrombogenic materials will be the key constituents not only of the vascular access, but also of the circuit and the dialyser membranes. This circuit system must guarantee the satisfactory performance of all safety systems. Monitoring of ongoing therapy and management of therapeutic prescription should be done remotely through a software solution. Dialyzer dimensions should be reduced and membranes should perfectly mimic physiological functioning of a nephron. Since the dialysate can be continuously regenerated and reused, the amount of dialysate should be lesser than 500 cc while simultaneously having a high adsorption capacity of small and middle size molecules. Given the continuous functioning of the system, large amounts of energy are required. The implementation of energy-efficient and cost effective batteries and fuel cells should be considered.33
The WAK/WUF pump system should evolve to meet some key needs such as high safety rates, elevated biocompatibility, and should allow for fluxes compatible with patient's well-being, minimal hemolysis and costs of production as controlled as possible.39
Importantly, the WAK/WUF system must be user-friendly to permit self-care; they must be wearable to allow mobility; they also must be affordable. All these characteristics can only be achieved through a joint collaboration of multidisciplinary teams consisting of nephrologists, engineers and economists.
ConclusionCKD is one of the twenty leading causes of death worldwide. Its’ prevalence has almost doubled between 1990 and 2010 and is expected to continue to increase. Despite the great technical and clinical evolution in RRT, the morbidity and mortality of ESRD patients remain too high and their quality of life precarious. RRT should evolve to match clinical needs such as greater solute clearance, better volume control, improved nutritional status and to reduce ESRD complications. Importantly they must allow a higher health-related quality of life with minimal restrictions in ADL and remove the physical barriers associated with the current available techniques. The economic aspects associated with CKD must also be taken into account. Thus, it is imperative to develop new RRT techniques that fit each patient clinical and personal needs. In this way, the use of a fully portable device, such as WAK and WUF can be a very interesting option for some patients with ESRD that should be considered.
WAK and WUF still requiring considerable technological improvement in the currently used dialysis equipment, in terms of safety, biocompatibility and portability.32 Importantly, the development of an appropriate vascular access as well as a good pumping system are also crucial, since it may allow high safety, low hemolysis, and low power consumption.47
Conflict of interestsNo conflicts of interest to declare.