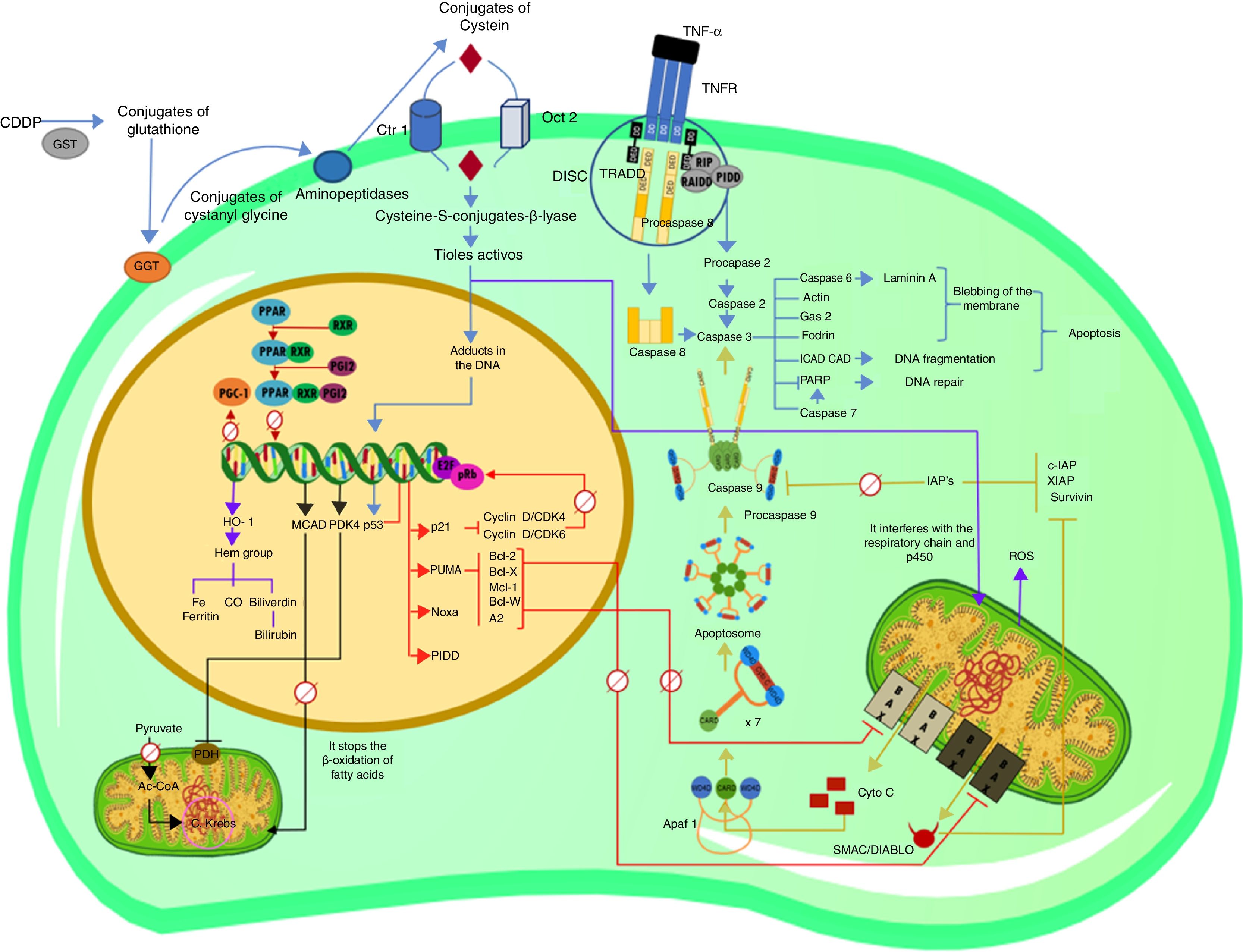
Cisplatin (cis-diaminodichloroplatin (II), CDDP) is an antineoplastic drug used in the treatment of many types of cancer of solid organs. Although CDDP side effects include ototoxicity, gastric toxicity, myelosuppression and allergic reactions, the main limitation of this antineoplastic agent is nephrotoxicity, provoking acute kidney failure (AKI). The pathophysiology of AKI induced by CDDP is complex, there are several mechanisms involved, including nuclear and mitochondrial damage, activation of apoptosis signaling pathways, generation of reactive oxygen species, and inflammation. In this review, we describe the molecular events of CDDP, which cause nephrotoxicity, affecting the expression of several genes involved in metabolism and activating different apoptotic signaling pathways, highlighting the main cellular targets of this antineoplastic and the approach of possible treatments for AKI caused by CDDP.
El cisplatino (cis-diaminodicloroplatino(II), CDDP) es un fármaco antineoplásico usado en el tratamiento de muchos tipos de cáncer en órganos sólidos. Aunque los daños por CDDP incluyen ototoxicidad, gastrotoxicidad, mielosupresión y reacciones alérgicas, la nefrotoxicidad es el principal efecto limitante de este antineoplásico, provocando comúnmente insuficiencia renal aguda (IRA). La fisiopatología de la IRA inducida por CDDP es compleja, ya que están involucrados diversos mecanismos como el daño nuclear y mitocondrial, la activación de vías de señalización de apoptosis, generación de especies reactivas de oxígeno y la estimulación de la inflamación. En esta revisión, se describen los eventos moleculares por los cuales el CDDP tiene su acción nefrotóxica al alterar la expresión de diversos genes que participan en el metabolismo y al activar diferentes vías de señalización apoptóticas, resaltando las principales dianas celulares de este antineoplásico y su uso para el planteamiento de posibles tratamientos para la IRA causada por CDDP.
Cisplatin (cis-diaminodichloroplatin (II), CDDP) is an antineoplastic drug used in the treatment of many types of cancer of solid organs, including cancer of the head and neck, lung, testicle, ovary and breast.1
Clinical studies have shown that approximately one third of patients who use this antineoplastic agent experience acute renal injuries after treatment with CDDP, presenting a reduction in glomerular filtration rate with increased blood urea nitrogen and creatinine, together with electrolyte imbalance.2
Although CDDP side effects include ototoxicity, gastric toxicity, myelosuppression and allergic reactions, the main limitation of this antineoplastic agent is nephrotoxicity. Although CDDP may have different harmful effects in the kidney, the most common is acute kidney injury (AKI).1
The AKI occurs in approximately one of 5 adults and in one of 3 children hospitalized with acute illness, presenting an unacceptably high mortality. The increase in severity of AKI is associated with increased mortality, which occurs mainly in patients who require renal replacement therapy.3
In this review, we describe the molecular events by which CDDP produces nephrotoxicity, affecting the expression of several genes involved in metabolism and activating different apoptotic signaling pathways (Fig. 1). The main cellular targets of this antineoplastic and the approach of possible treatments for AKI caused by CDDP are highlighted.
Explanatory diagram of the apoptotic pathways and alterations in gene expression in cisplatin-induced nephrotoxicity (CDDP).
Process by which CDDP enters the cell and forms adducts with DNA, causing the expression of p53, as well as the extrinsic pathway of apoptosis and the activation of procaspase 2. Molecular apoptotic events that are triggered by the activation of p53, as well as changes in the transcriptional activity of PPAR and its co-activator PGC-1. Intrinsic or mitochondrial pathway of apoptosis before the activation of caspase 3. Transcriptional alterations in target genes of PPAR; decreased expression of MCAD and increased expression of PDK4. Interactions of the CDDP that trigger the overexpression of HO-1 by the increase in ROS.Ac-CoA: acetyl-coenzyme A; AIP: apoptosis inhibitor protein; Apaf 1: apoptosis protease-activating factor-1; C Krebs: Krebs cycle; CAD: caspase-activated deoxyribonuclease; CARD: caspase recruitment domain; CDDP: cisplatin; CO: oxide carbon; Ctr1: copper transporter 1; DD: death domain; DED: death effector domain; DISC: death-inducing signaling complex; E2F: E2-factor; GAS-2: growth arrest specific protein 2; GGT: gamma glutamyl transpeptidase; GST: glutathione-S-transferase; HO-1: heme oxygenase 1; ICAD: inhibitor of caspase-activated deoxyribonuclease; MCAD: medium chain acyl dehydrogenase; OCT2: organic cation transporter 2; PARP: poly(ADP-ribose) polymerase; PDH: pyruvate dehydrogenase; PDK4: pyruvate dehydrogenase kinase isozyme 4; PGC-1: PPAR-gamma-coactivator-1; PGI2: prostacyclin; PIDD: p53-induced protein with a death domain; PPAR: peroxisome proliferator-activated receptor; pRb: retinoblastoma protein; PUMA: p53 upregulated modulator of apoptosis; RAIDD: RIP-associated Ich-1/Ced-3-homologue protein with a death domain; ROS: reactive oxygen species; RXR-α: retinoid X receptor; SMAC/DIABLO: second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI; TNF-α: tumor necrosis factor alpha; TNFR: TNF receptor; TRADD: tumor necrosis factor receptor type 1-associated death domain.
After the administration of CDDP, two thirds of the compound binds quickly and irreversibly to plasma through glutathione, methionine, cysteine, albumin and nucleotides, forming inactive CDDP complexes. The one third is excreted by kidneys by glomerular filtration and tubular secretion through active transport mediated by OCT2 (organic cation transporter 2) and by the transporter for extraction of multiple drugs and toxins.4
However, it has been reported that one hour after the administration of CDDP, the concentration of CDDP in plasma does not change, its elimination by the renal route is scarce. The half-life of the CDDP in the kidney is very long, ranging from 100 to 300h, this is because the distribution of the drug in the renal epithelial cells is through a covalent union with the protein forming CDDP-protein complexes, which can not be eliminated, contributing to nephrotoxicity over a long period of time. In fact, in vivo studies have shown that histological and biochemical damage are more evident 5 days after administration of CDDP.5–7
Affinity of renal cells to cisplatinThe nephrotoxicity of CDDP has been attributed to high concentrations in the kidneys and in the renal transport system. During the excretion process, blood levels of CDDP are generally non-toxic, however, the amount of CDDP in tubuloepithelial cells can be up to 5 times higher than in the blood, reaching toxic levels. The high concentrations of CDDP in the kidneys favor passive diffusion into cells, the main mechanism whereby CDDP accumulates in the cell. However, it has been recently reported that the active transport system associated to nephrotoxicity is related to the membrane transporters OCT2 and Ctr1 (copper transporter 1).8
OCT2 is highly expressed in the S3 segment of the proximal tubules, where the nephrotoxic effect of CDDP9 takes place, whereas Ctr1 is expressed largely in the adult kidney in the basolateral membrane of the proximal tubule, being a transporter of copper that is also capable of mediating the uptake of CDDP in mammalian cells.1 In addition to the transport facilitated by OCT2 in the basolateral membrane of the proximal tubule, 60% of the CDDP is recovered by the lysosome system, indicating that the vesicle cycle plays a crucial role in the accumulation of CDDP in the tubulo-epithelial cells. As mentioned, the proximal tubular cells contain up to 5 times more CDDP than blood, making this region more susceptible to injury than other parts of the nephron such as the loop of Henle, which also presents damage.5,10,11
A study by Kishore et al. reveals that CDDP causes a significant decrease in the expression of aquaporin 1 (AQP1) in the descending branch of the loop of Henle, causing a reduction in the rate of water absorption and polyuria.12 In addition, there is evidence that the function of the loop of Henle may be affected by an imbalance in magnesium homeostasis, or by the reduction of Mg membrane transporters.13
Moreover, this antineoplastic forms glutation conjugates in blood circulation through the action of GST (glutathione-S-transferase), giving rise to the formation of a potent toxin. Once this conjugates are inside of the proximal tubule cell, the cysteine conjugates are further metabolized by the action of the S-conjugated cysteine beta-lyase to thiols that are highly reactive.1
With all the aforementioned, It can be inferred that the proximal convoluted tubules are the main site of CDDP damage, this is due to the high expression of enzymes involved in their metabolism1 and the expression of transcription factors that regulate the energy metabolism of the kidney.14 Thus there are a more severe morphological abnormalities in the proximal convoluted tubule than in the loop of Henle.
Mechanisms of cisplatin nephrotoxicityThe pathophysiology of AKI induced by CDDP is complex, there are several mechanisms involved including nuclear and mitochondrial damage, activation of apoptosis signaling pathways, generation of reactive oxygen species (ROS) and inflammation.7,15
The CDDP is formed by a platinum ion bound to 2 chloride ions and 2 ammonium molecules. The cytotoxicity of CDDP is related to reactive aqueous metabolites, which are formed depending on the concentration of chloride ions. The intracellular chloride concentration is 20mM, which is lower than the blood concentration, 100mM, so the CDDP remains without alterations in the bloodstream, but is hydrolyzed in the intracellular environment, replacing the chloride ions by water molecules generating a positively charged electrophile. Therefore, in its aqueous form, CDDP produces [Pt(NH3)2Cl(OH2)]+ and [Pt(NH3)2(OH2)2]2+8. The platinum atom forms covalent bonds with the N7 positions of the purine bases to form intrachain linkages, mainly 1,2 or 1,3, and a smaller number of interchain links, stopping replication, transcription and the cell cycle which eventually leads to apoptosis of cells with a high proliferation index, while cells with a low rate of proliferation are relatively protected from this damage; however, proximal tubule cells are an exception, being damaged by CDDP.8,16,17
Apoptosis induced by DNA damage is mediated by the p53 tumor suppressor gene. In adult humans, cells of the proximal tubules do not replicate, so the formation of adducts with DNA does not play a relevant role in the nephrotoxicity of CDDP. However, it has been suggested that mitochondrial DNA, or other mitochondrial targets, are perhaps more important than the nuclear DNA damage in the cell death induced by CDDP.8 To produce these effects, the CDDP hydrolyzes producing a positively charged metabolite that accumulates preferentially within the negatively charged mitochondria. Therefore, the sensitivity of the cells to CDDP seems to correlate with the density of mitochondria and the membrane potential of the mitochondria. This may explain the sensitivity of the renal proximal tubule to the toxic effect of CDDP, since this segment has very high densities of mitochondria as compared with the rest of the nephron.1
The oxidative damage and inflammatory events induced by CDDP may also explain the nephrotoxic effects in other cellular components. Several studies indicate that the nephrotoxicity of CDDP is associated with the generation of ROS,8 and decrease the antioxidant mechanisms.5 This generation of ROS amplifies cell death mediated by the Fas ligand (FasL)/Fas. This apopotosis pathway is considered one of the pathways involved in kidney damage, where the Fas receptor and its ligand translate apoptotic signals by activating caspase 3 and 8 effectors. Nevertheless, current studies suggest that CDDP does not increase levels of soluble FasL in blood, it increases only in renal tissue with the release of FasL from membrane by still unknown mechanisms. The increases in metalloproteinases activity mediated by ROS, may contribute to the breakdown of membrane FasL generating soluble FasL.18,19
Additionally, CDDP increases tumor necrosis factor α (TNF-α), nitric oxide, adhesion molecules and CD4+ T cells, inducing a 6 fold increase in inflammatory cytokines such as interleukin 1-β, interleukin 18 and interleukin 6, that causes kidney damage.5
Finally, another of the mechanisms that may contribute to the nephrotoxicity caused by CDDP is the inhibition in the synthesis of mitochondrial energy compounds. Fatty acids are the main source of energy for the proximal tubule and the primary site of CDDP-induced kidney injury. CDDP inhibits the oxidation of fatty acids in the mouse kidney and in proximal tubular cells in culture. This is caused by a reduction in the expression of genes involved in the use of cellular fatty acids mediated by PPAR-α (peroxisome proliferator-activated receiver).14
The role of PPAR-α (peroxisome proliferator-activated receptor) in the nephrotoxicity induced by cisplatinPPARs are receptors of nuclear hormones, that is, ligand-dependent intracellular proteins that stimulate the transcription of specific genes by binding to specific DNA sequences. When activated by binding to a ligand, its transcription factors exert an effect on growth and metabolism. There are 3 subtypes of PPAR which are the products of different genes, commonly designated as PPAR-α, PPAR-γ and PPAR-β/δ, or simply δ.20
The subtype PPAR-α is involved in the regulation of renal metabolism and it maintains the balance between energy production and expenditure. It is highly expressed in the proximal tubules cells.20 PPAR-α forms a heterodimer with RXR-α (retinoid X receptor) which binds to a specific DNA sequence, called PPRE (PPAR response element), located upstream of target genes that are mainly involved in the oxidation of fatty acids.21
The PPREs consist of a consensus sequence of AGGTCA hexanucleotides spaced by a single nucleotide. The agonist ligands for PPAR promote the interaction of the PPAR-RXR heterodimer with DNA; however, the binding of PPAR with DNA may occur at a basal state.22
In contrast to other pairs of RXR nuclear receptors, the PPAR-RXR heterodimers are “permissive”, which means that they can be activated by a selective ligand of either RXR or PPAR. Binding of the ligand leads to the dissociation of the corepressor proteins and the association of coactivator proteins, which may have or just incorporate an intrinsic histone deacetylase and histone acetyltransferase activity, necessary for the assembly of the transcription initiation complex.22
Therefore, PPAR-α forms a heterodimer with RXR. In the absence of ligands, the dimer may recruit a corepressor, thus inhibiting the transcription of targets genes mediated by PPAR; however, the presence of an agonist or activator, such as prostacyclin (PGI2), triggers the recruitment of a coactivator complex that induces the transcriptional activity of PPAR-α on its target genes. This leads to an increase in the catabolism of fatty acids and the production of adenosine triphosphate (ATP), and also contributes to reduce products of peroxidation of cytotoxic fatty acids which helps the viability of renal epithelial cells.20
Target genes of PPAR-α (peroxisome proliferator-activated receptor)One target gene of PPAR-α is the one that encodes for MCAD (medium chain acyl dehydrogenase), a homotetramer that catalyzes the initial step of the mitochondrial β-oxidation of fatty acids of 4–12 carbons. This step consists in the dehydration of acyl-CoA in positions C2 and C3. As a result, an unsaturated delta-enoyl-CoA derivative with a double bond in the trans position is obtained. The 2 hydrogen atoms are transferred by the MCAD, (which contains flavin adenine dinucleotide (FAD)), to the electron transfer flavoprotein (ETF). These are in turn transferred to ubiquinone (coenzyme Q), a component of the respiratory chain. Therefore, the degradation of fatty acids undergoes a series of oxidation reactions until they form fatty acids of 2 carbons.23
A study by Li et al., in 2004, demonstrated that CDDP causes a significant reduction of both, mRNA levels and enzymatic activity of MCAD, in kidneys of adult mice. However, the use of the WY, a ligand for PPAR-α, reduced AKI-induced by CDDP by preventing the reduction of mRNA levels and increasing the enzymatic activity of MCAD. This data demonstrates that ligand-mediated PPAR-activity contributes positively to the restoration of renal function and the transcription of genes involved in the metabolism of fatty acids.14
One of the possible explanations for the reduction of MCAD mRNA levels due to CDDP-induced nephrotoxicity is that this antineoplastic agent is capable of inhibiting the expression of PGC-1 (PPAR-gamma-coactivator-1) in proximal tubular cells and in the ascending portion of the loop of Henle of the mouse kidney. PGC-1 is the most important co-activator of PPAR-α in the kidney; in situ hybridization studies have demonstrated the expression of PGC-1 in these 2 segments of the nephron, which also express high levels of PPAR-α and enzymes involved in the oxidation of fatty acids.21 Therefore, the inhibition in the expression of PGC-1 means a reduction of the transcriptional activity of PPAR-α with the consequent decrease in mRNA levels of target genes such as MCAD. However, this mechanism of transcriptional regulation does not fully explain the changes in the expression levels of other genes that participate in important metabolic processes of the kidney, such as glycolysis. Li et al. showed that in mice, CDDP is able to increase expression of PDK4 mRNA and protein (pyruvate dehydrogenase kinase isozyme 4), which represents a decrease in the oxidation of carbohydrates, and that the use of the PPAR-α ligand, WY re-establishes basal mRNA levels of PPAR-α.14 Surprisingly, in other studies on muscle cells of PPAR-α knockout mice, the increase in PGC-1 induced activation in the expression of the PDK4 gene, demonstrating that the expression of this gene may be independent of PPAR.24 There is no consensus on the mechanism of regulation of genes involved in glucose metabolism such as PDK4, at least not on the role that PGC-1 and PPAR-α may have in the transcription of these genes.
Heme oxygenase-1 in response to nephrotoxic damage of cisplatinThe increase in ROS is one cause of AKI. Kidneys are particularly vulnerable to free radicals, because it is one of the prominent organs for oxidative processes.25 CDDP can induce the generation of various ROS through several mechanisms, the inactivation of the cellular antioxidant system, interruption of the mitochondrial respiratory chain, interaction with the microsomal cytochrome P450, amplification of cell death mediated by FasL or increasing metalloproteinases.18,19,26
It has been proven that in high states of oxidative stress, the expression of the enzyme heme oxygenase-1 (HO-1) is induced as a protective response of cells exposed to a diverse range of toxic factors.25
The HO-1 catalyzes the step limiting rate of degradation of the heme group. The products of degradation are carbon monoxide, iron and biliverdin. In a subsequent reaction, biliverdin reductase (EC 1.3.1.24) produces bilirubin from biliverdin.27
Although the cellular processes underlying the induction of HO-1 are complex and tightly regulated, a common denominator for most of these stimuli is a significant change in cellular redox.25 In toxic nephropathy by CDDP, in a model of acute oxidative stress that does not directly depend on the administration of heme proteins to the kidney, HO-1 increases in renal tubules 6h after CDDP administration.28 Likewise, Shiraishi et al. in 2000 demonstrated administration of CDDP to transgenic mice deficient in HO-1 (−/−) develop renal failure faster with a significantly more severe renal lesions than the wild-type (+/+) treated mice.25
p53, an important mediator in cell death induced by cisplatinOne of the CDDP's cellular targets is nuclear DNA. The interaction of this antineoplastic drug with the genetic material induces nephrotoxicity involving multiple molecules and signaling pathways.
The molecule p53 has gained attention in recent years as a potent inducer of apoptosis as a mechanism of CDDP induced nephrotoxicity. The p53 tumor suppressor gene induces cell cycle arrest or apoptosis in response to DNA damage, oncogene activation and hypoxia.
There is a vast amount of information gathered in multiple investigations, showing evidence on the participation of this protein in the mechanisms of CDDP induced cell death. One of these investigations was carried out by Cummings and Schnellmann in 2002, where it was shown by immunofluorescence, an increase in the nuclear expression of p53 in rabbit proximal tubule cells 4h after administration of CDDP.29
Another study by Jiang et al. in 200430 confirms the participation of p53 in CDDP nephrotoxicity using in vitro models of rat proximal tubular cell cultures; the study demonstrated that p53 is rapidly phosphorylated and positively regulated after treatment with CDDP, before the onset of cellular apoptosis. The activation of p53 was not inhibited by carbobenzoxy-Val-Ala-Asp fluoromethyl ketone, nor by Bcl-2 (B cell lymphoma 2), although both suppressed CDDP-induced cellular apoptosis. This indicates that p53 activation was not a consequence of cell death. Pifitrin-α blocked the activation of p53 attenuating caspase activation and apoptosis. The results suggested that p53 activation may be an early signal for renal tubular cell apoptosis induced by CDDP. Likewise, apoptosis induced by CDDP was inhibited in a mouse with a point mutation at the p53 DNA binding site, demonstrating that the proapoptotic effects of p53 in response to CDDP depend to a large extent on its transcriptional activity of target genes.
Three years later, Wei et al. demonstrated by immunoanalysis an increase of p53 protein in renal cortex and medulla samples after treatment with CDDP in C57BL/6 mice.31 The increase in this protein was evident from day 2 and intensified at day 3. Parallel to the accumulation of p53, there was phosphorylation of p53 that was accompanied by the development of AKI. This analysis by immunofluorescence showed the accumulation of p53 and its phosphorylation mainly in the nucleus of the renal cortical cell. In this same study, it was also shown that the CDDP-induced nephrotoxicity decreased significantly in mice deficient for the p53 gene. Compared with animals of the wild strain, mice deficient in p53 showed better renal function, less tissue damage and fewer apoptotic cells.
Reactive oxygen species and p53 in the induction of apoptosis mediated by cisplatinExperimental studies show that free radicals from oxidative stress cause the cisplatin induced nephrotoxicity; these free radicals have an effect on reabsorption of water, sodium and glucose in the proximal tubule. The study by Ju et al. in 2014, allows to expand on the apoptotic pathway; they demonstrated that in rat mesangial cells the treatment with CDDP results in the activation of p53, as well as an increase in intracellular ROS levels. Interestingly, 2 well-known antioxidants (N-acetylcysteine and dimethyl thiourea) significantly reduced apoptosis induced by CDDP, suggesting that ROS production, together with activation of p53, are involved in the generation of apoptosis by CDDP.32
Considering that both ROS and p53 were involved in the induction of apoptosis mediated by CDDP, one can infer interactions between both. It has been established that ROS act as mediators of p53-induced apoptosis, however, recent evidence revealed that ROS can also act as an upstream signal that triggers the activation of p53. This suggests that there is a positive feedback between p53 and ROS, in addition to the toxic effects of CDDP causing inactivation of glutathione and related antioxidants32; thus the cellular redox state is also modified by interaction with proteins from the mitochondrial respiratory chain such as p450, as it was previously mentioned.26
Additionally, it is important to mention the molecules by which p53 can trigger its apoptotic effects and, particularly, the studies that have demonstrated these effects in the nephrotoxicity caused by CDDP.
Transcriptional regulation of p53 in cisplatin-induced nephrotoxicityThere are several genes that contain promoters with p53 binding sites; these genes may undergo transcriptional activation, or, otherwise they may be repressed. Some of these genes have apoptotic activity through various signaling pathways, and several of them are regulated by p53 during the CDDP induced nephrotoxicity.
PUMA (p53 upregulated modulator of apoptosis)The proteins of the Bcl-2 family are evolutionarily conserved and they are key regulators of apoptosis. PUMA (p53 upregulated modulator of apoptosis) is one of the members of this family of proteins, which in turn belongs to a subfamily of proapoptotic proteins that have only BH3 domain, which corresponds to an amphipathic α-helix that binds directly to the proteins of the antiapoptotic Bcl-2 family. PUMA activity seems to be controlled exclusively by transcription, while other BH3 proteins are often activated through multiple mechanisms, including post-transductional modifications. PUMA is transactivated by p53 in response to genotoxic stress, such as DNA damage. Basically PUMA represents all the proapoptotic activity of p5333 together with another BH3 protein, Noxa, which in most cases has a more limited function.
This transactivation occurs within a few hours after DNA damage has occurred, and begins with the recruitment of p53 by the 2 response elements of p53 in the PUMA promoter. Gene targeting studies have indicated that both p53 and p53 binding sites in the PUMA promoter are indispensable for the induction of PUMA after DNA damage. The binding of p53 to the PUMA promoter facilitates the modifications of central histones, such as the acetylation of histones H3 and H4, which leads to the opening of the chromatin structure and activation of transcription.33
Like other BH3 proteins, PUMA is a signaling molecule that transduces signals of death to mitochondria acting through multiple members of the Bcl-2 family to induce mitochondrial dysfunction and caspase activation. PUMA acts mainly through indirect activation of Bax and/or Bak by inhibiting antiapoptotic Bcl-2 family member proteins, including Bcl-2, Bcl-XL, Mcl-1, Bcl-w and A1. It has also been suggested that PUMA can trigger apoptosis through direct activation of Bax, or cytoplasmic p53 in some cells.33
In relation to CDDP-induced nephrotoxicity, it has been shown that PUMA-α, but not other isoforms, is remarkably induced by CDDP in both in vitro models of cultured proximal tubular cells and in vivomouse kidneys. The induction of PUMA-α by CDDP is p53 dependent, because pifitrin-α, an inhibitor of p53, inhibites the PUMA-α expression and the same occurred in p53 knockout mice. Likewise, it was demonstrated that PUMA-α accumulated in the mitochondria after its induction, where it interacted with Bcl-xL to release proapoptotic molecules such as Bax. Activated Bax permeabilizes the mitochondrial membrane with the release cytochrome C, resulting in the activation of caspases and causing apoptosis. Thus, both in vitro and in vivo experiments suggested the involvement of PUMA-α in p53-mediated renal cell apoptosis during the nephrotoxicity of CDDP.34
PIDD (p53-induced protein with a death domain)The function of PIDD (p53-induced protein with a death domain) is highly controlled by several mechanisms, allowing the generation of different fragments with specific functions. The expression of PIDD is regulated at the transcriptional level and by alternative splicing, while its function is further regulated by autoproteolytic cleavage, and through intra- and intermolecular interactions with proteins. PIDD expression can be induced by p53 or by inhibition of the apoptosis inhibitor BRUCE. However, even at a basal situation, no correlation could be found between the p53 status and the PIDD expression level, showing that at least the basal expression of PIDD is independent of p53.35
At the post-transductional level, different PIDD fragments are generated with specific functions: PIDD-C is the fragment that participates in functions of cell survival and repair (activation of TLS and NF-κB), while PIDD-CC joins RAIDD (RIP-associated Ich-1/Ced-3-homologue protein with a death domain) and initiates the activation of caspase 2, and forming a complex of approximately 700kDa, called PIDDosome.35
In proximal tubular cells culture, PIDD is capable to mediate the apoptosis induced by CDDP.36 In these cells, PIDD was induced by CDDP and it was attenuated by pifitrin-α and micro RNA interfering with p53, suggesting a p53-dependent mechanism. After the induction of PIDD, caspase 2 is activated leading to mitochondrial release of AIF (apoptosis inducing factor), resulting in chromatin condensation, nuclear DNA degradation and apoptosis of tubular cells.
CaspasesCaspases are a family of cysteine proteases that are found as inactive precursor molecules (procaspases), and after receiving an apoptogenic signal (such as TNF-α) suffer a proteolytic breakdown and give rise to 2 subunits that constitute the active enzyme (caspase). The procaspases consist of an N-terminal prodomain and 2 subunits, a large p20 and a small p10. To date, the most studied caspases are 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10. Taking into account the primary structure determined by nuclear magnetic resonance and X-ray crystallography, they can be grouped in 2 classes: class I are procaspases with a large N-terminal prodomain, such as procaspases 1, 2, 8, 9 and 10; the class II procaspases have a small N-terminal prodomain or even lacks the N-terminal prodomain, the procaspases 3, 6 and 737 belong to this class.
The prodomains of the class I procaspases contain protein–protein interaction domains that favor the formation of homodimeric complexes. These complexes are composed of tetramers of 2 large and 2 small subunits that favor their self-proteolytic cleavage at specific sites of aspartic acid residues (D). All these molecules consist of 6 α-amphipathic and antiparallel helices which are packaged forming a hydrophobic center, causing hydrophobic interactions between the DEDs (death effector domains) and electrostatic interactions between the CARDs (caspase recruitment domains). The self-activation of class I caspases guides them to the cytoplasm to activate class II procaspases, which do not have the capacity of self-proteolysis and, during their activation, they form heterodimeric complexes that favor proteolytic breakdown. Under these conditions, active caspases initiate the apoptotic mechanisms by hydrolyzing the various cytoskeletal proteins, nuclear proteins, proteins that intervene in cell division, cell cycle control, DNA repair, replication and transcription.37
There are 2 main cascades for the execution of apoptosis. The first one takes place in the cell membrane, and depends on the action of the so-called death factors acting through receptors resulting in the formation of a DISC (death-inducing signaling complex). This DISC generates a signal that recruits initiator caspases in the cell membrane. A variation of this cascade is the deprivation of growth factors, which also generates signals from the membrane to the mitochondria. The second cascade involves the mitochondria, the key organelle that integrate intra- and extracellular signals of apoptosis.37 This activation pathway takes place with the Bax and/or Bak dimerization, which allows the exit of proapoptotic molecules such as SMAC/DIABLO (second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI) or Cyto C (mitochondrial cytochrome c), that through its interaction with Apaf 1 (apoptotic protease activating factor 1), displaces CARD, being located in the middle of the 2 WD40 domains of Apaf 1, causing its activation and initiating the formation of apoptosome which is composed by 7 activated Apaf 1 molecules. This, in turn, facilitates the activation of procaspase 9, which in turn activates procaspase 3.
Regarding the transcriptional regulation of caspases by p53, it has been discovered that caspases 1, 6, 7 and 10 have specific binding elements to p53 in their promoter regions, and can be activated by p53.33
Yang et al., in 2007, proposed a p53-dependent transactivation of caspases 6 and 7 in CDDP nephrotoxicity, demonstrating that specific binding of CDDP to DNA sequences produce overexpression of p53 with a resulting increase expression of caspases 6 and 7, as well as their activities in renal tubular cells and the renal cortex, while the inhibition of p53 with the use of pifitrin-α blocked the activation of both caspases in tubular cells, canceling CDDP induced apoptosis, tissue damage and renal dysfunction.38
The activation of the different caspases that mediate apoptosis, such as caspases 3, 6 and 7, may cleave cellular proteins such as: PARP (poly(ADP-ribose) polymerase) which is involved in DNA repair;laminin A and fodrin, which are essential components of the nuclear and cytosolic skeleton respectively; CAD (caspase-activated deoxyribonuclease) which is a constitutive type of magnesium-dependent endonuclease that can be activated by caspases, playing an important role in the degradation of DNA in mammalian apoptosis. In normal cells, CAD resides in the nucleus forming a complex with its specific inhibitor, ICAD; and finally, GAS-2 (growth arrest specific protein 2), which is cleaved during apoptosis and the excised form induces intense rearrangements of the actin cytoskeleton.39
p21p21Waf1/Cip1/Sdi1 is the first identified inhibitor of the cyclin/CDK complexes, which regulate the transitions between the different phases of the cell cycle; it belongs to the Cip/Kip family. In addition to regulating the cell cycle, Cip/Kip proteins play an important role in apoptosis, transcriptional regulation, cell migration and cytoskeletal dynamics. A complex phosphorylation network modulates the functions of Cip/Kip proteins altering its subcellular localization, protein–protein interactions and stability.40
The progression of the cell division cycle is regulated by the coordinated activities of the cyclin/CDK complexes. One way of regulating these cyclin–CDK complexes is through their binding to CDK inhibitors (CKI). In the metazoans, 2 families of CKI genes have been defined; the INK4 gene family, where p16INK4a, p15INK4b, p18INK4c and p19INK4d are found, which bind to CDK4 and CDK6 and inhibit their kinase activities by interfering with their association with type D cyclins. On the contrary, the CKIs of the Cip/Kip family binds to the cyclin and CDK subunits, and can modulate the activities of the complexes cyclin D, E, A, and B with CDK. The members of the Cip/Kip family are p21Cip1/Waf1/Sdi1 (p21 encoded by cdkn1a), p27Kip1 (p27, cdkn1b) and p57Kip2 (p57, cdkn1c), which share a conserved N-terminal domain, that mediates the binding to cyclins and CDK.40
In addition, p21 is an important transcriptional target of phosphorylated p53, and it is mainly responsible for cell cycle arrest induced by DNA damage in G1. In addition, it inhibits the complex cyclin D with CDK4 and CDK6, so that pRb (retinoblastoma protein) cannot be phosphorylated and remains attached to the transcription factor E2F, which is maintained blocked.23 However, interestingly, the role of p21 is not limited to the progression of the cell cycle, it is also involved in the mechanisms of CDDP induced cell apoptosis.
Megyesi et al., in 1996, reported that after CDDP induced AKI in mice, kidney p21 mRNA is rapidly synthesized up to high levels.41 This study was performed using p53 knockout mice, demonstrating that activation of the p21 gene occurred through a p53-independent pathway. Also, in 1998, Megyesi et al. performed another study in mice homozygous for a deletion in the p21 gene; after administration of CDDP and as compared to the wild strain, the p21 knockout mice showed a faster onset of AKI with more severe morphological changes and higher mortality.42
Duration in the induction of the apoptotic processWei et al. showed a significant amount of p53 up to day 3 after CDDP administration in C57BL/631 mice.31 Likewise, other studies have reported that the expression of caspases 3 and 6 in cultured cells is maintained up for 7 days after the induction of apoptosis; although this was not induced with CDDP, this study demonstrates that the apoptotic process may extend beyond a week.43
It is worth highlighting the work of Eads et al. in 2016 that evaluated the pharmacokinetics of CDDP in patients with esophageal cancer and renal damage. They reported that CDDP can be detected in plasma in its active form up to 20 days after its administration. The clearance of ultrafiltrable platinum (platinum that is not bound to any macromolecule) in these patients with low glomerular filtration rate was similar to normal renal function patients.44
The evidence showing that the apoptotic process can be maintained for several days and the fact that the limited clearance allows CDDP to circulate for several days, explains that tubular cell apoptosis is maintained for days or weeks after the administration of CDDP.It has been reported that the cisplatin, carboplatin, as well as aminoglycosides, accumulate in proximal tubular cells causing AKI 5-7 days after its administration.13
It is known that CDDP is maintained in the bloodstream for up to 3 weeks, and the expression of molecules related to the apoptotic process has a half-life of little more than a week, however no studies have been conducted to directly correlate levels of CDDP with molecules involved in cell death processes.
Renoprotective strategies against the effects of cisplatinThere is enough information demonstrating the different effects of CDDP on the kidney at the molecular level; this is the basis for the search for new possible treatments and strategies to reduce renal damage caused by antineoplastic drugs.
Many studies have shown that the decrease in expression levels and transcriptional activity of PPAR-α is related with the development of CDDP induced AKI; and, that PPAR-α ligands, such as WY14643, re-establish the normal structure and function of the kidney.14 Other studies showed that PGI2 can act as an inducer of PPAR-α, enhancing the translocation of PPAR-α to the nucleus and the binding to the inflammatory transcription factor NFκB, thus inhibiting the apoptosis induced by TNF-α in renal epithelial cells.45 There is even evidence at the clinical level, and from experiments in rodents, suggesting beneficial effects of PPAR-α activation in diabetic nephropathy.46
The expression of HO-1 is related to the increase in ROS caused by the oxidative stress induced by CDDP. In vitro studies in human renal proximal tubule cells demonstrate that hemin, an inducer of HO-1, significantly attenuated CDDP-induced apoptosis and necrosis, whereas inhibition of HO-1 enzyme activity reversed this cytoprotective effect.25 Considering this data, it could be designed strategies for gene expression directed to HO-1, as well as the development of new physiologically relevant inducers of the endogenous HO-1 gene as a therapeutic and preventive modality in situations of high-risk AKI.
Likewise, given the effects of p53 on many genes that trigger apoptosis, there have been designed different pharmacological inhibitors of p53, such as pitfirin-α, a potent inhibitor of p53,30,33,36,38 or the α-2-(2-imino-4,5,6,7-tetrahydrobenzothiazol-3-yl)-1-p-tolylethane (PFT).29 There are also inhibitors of PUMA, such as the protein ABT-737 which contains a binding site to the BH3 domain of the proapoptotic proteins of the Bcl-2 family which is neutralized by binding to their hydrophobic surface grooves.47 And since PUMA represents practically all the proapoptotic activity of p53, it is better to use inhibitors against this protein, since some inhibitors for p53 usually have adverse side effects.33 However, antisense oligodeoxynucleotides are being developed, which contain enhancer elements that, when penetrated into cells, bind to DNA at specific sites of protein binding and thus interfere with genetic transcription in vitro and in vivo. This represents a powerful tool for gene manipulation, which can be directed to different organs to silence specific genes (E2F, p53, p21, among others) and avoid the side effects of chemotherapy or prevent the progression of diseases such as renal failure.
Finally, it would be advisable to continue the research on the nephrotoxic effects of CDDP in the loop of Henle, since it has been described its function may be affected by imbalance in magnesium homeostasis, or by the reduction of transporters of the cell membrane. Likewise, it is necessary to deepen the research on how soluble FasL may contribute in the local inflammatory process, whether it is proapoptotic or antiapoptotic.
ConclusionCDDP is an antineoplastic drug that acts at different molecular levels, being able to activate apoptotic pathways and stop important metabolic processes in the cell. The most important mechanism of action lies in its interaction with DNA, forming adducts that alter the transcription of genes involved in various cellular processes.
An strategy to diminish the side effects of compounds derived from platinum would contemplate the addition of a molecule to the platinum atom which would allow to interfere with a specific signaling pathway after binding to a specific protein, so its effects no longer lie only in the formation of DNA adducts.
There is still much to investigate, and the exact molecular mechanisms whereby diseases damage the organism remain an enigma. Learning what is the role of certain key molecules in the most important signaling pathways represents the challenge to be pursued so more specific and effective treatments can be developed.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Bernal-Barquero CE, Vazquez-Zapien GJ, Mata-Miranda MM. Revisión de las alteraciones en la expresión génica y vías apoptóticas provocadas en la nefrotoxicidad inducida por cisplatino. Nefrologia. 2019;39:362–371.