We investigated whether persistent antiphospholipid syndrome (APLAs) at diagnosis are associated with the risk of thrombotic events during follow-up in patients with ANCA-associated vasculitis (AAV).
MethodsWe retrospectively reviewed the medical records of 138 AAV patients. Thrombotic events were defined as arterial and venous thrombosis confirmed by magnetic resonance imaging, computed tomography, angiography and Doppler ultrasonography. Clinical and laboratory variables at diagnosis and during follow-up between patients with and without thrombotic events were compared. The univariable and multivariable Cox hazard model analyse to appropriately obtain hazard ratio (HR) considering the follow-up duration were conducted.
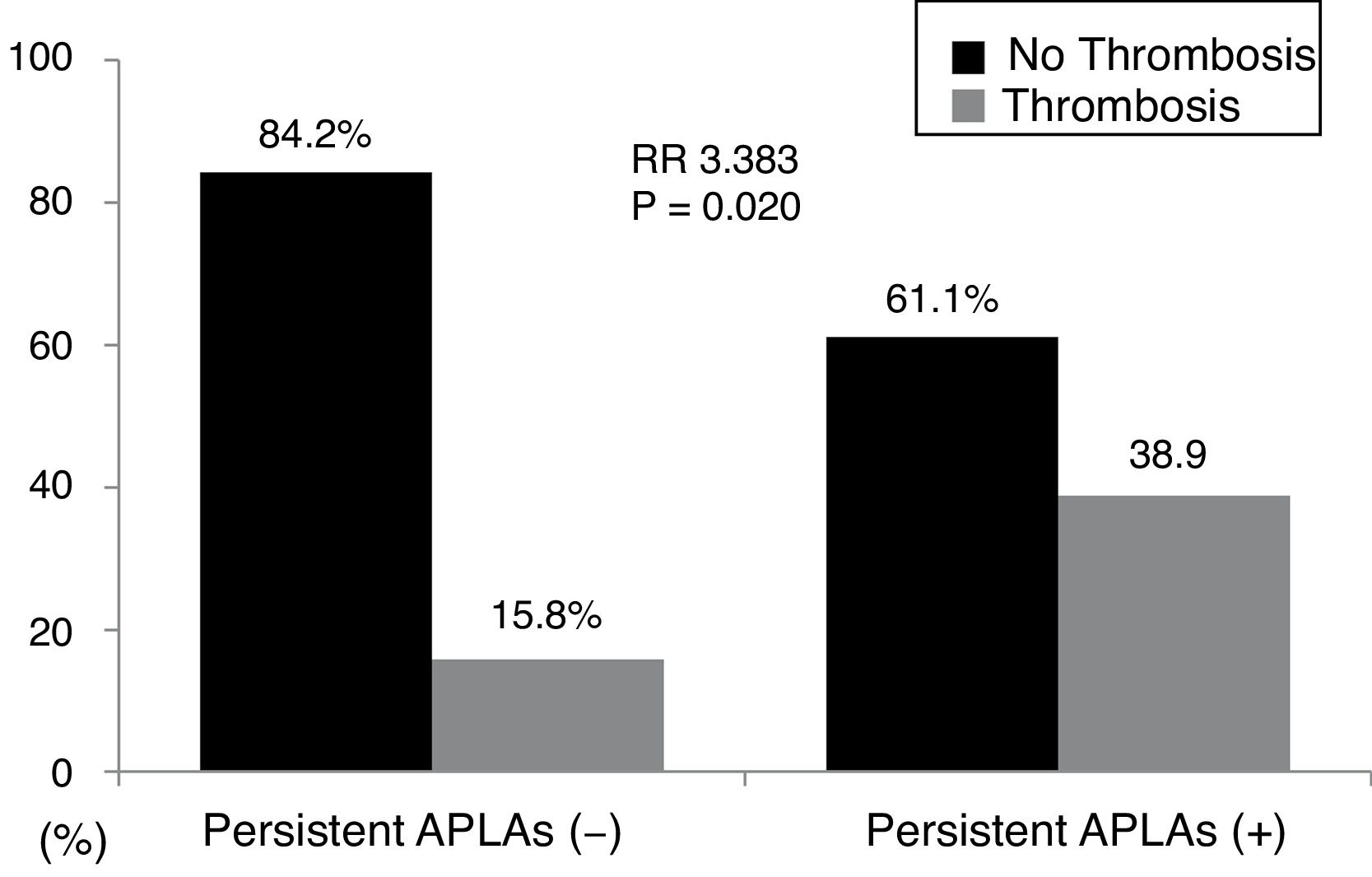
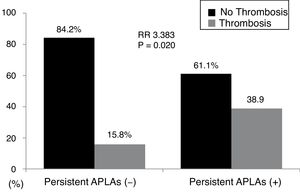
ResultsThe mean age of 138 AAV patients was 55.1 years and 44 were male. Persistent APLAs were detected in 18 patients with AAV (13.0%). Thrombotic events were observed in 26 patients with AAV (18.8%) during follow-up. At the time of a retrospective study point, persistent APLAs at diagnosis were observed more frequently in patients with thrombotic events than those without. In the multivariable Cox hazard model analysis, age at diagnosis (HR 1.075) and persistent APLAs (HR 2.902), but not ANCAs. Thrombotic events were identified more frequently in patients with persistent APLAs at diagnosis than those without (38.9% vs. 15.8%, relative risk 3.383).
ConclusionPersistent APLAs at diagnosis are significantly associated with the risk of thrombotic events during follow-up of AAV. We suggest that physicians should closely monitor the development of thrombotic events during follow-up.
Se investigó si el síndrome antifosfolipídico (SAF) persistente en el momento del diagnóstico se asocia a un riesgo de eventos trombóticos durante el seguimiento en pacientes con vasculitis asociada con ANCA (VAA).
MétodosSe revisaron de forma retrospectiva las historias clínicas de 138 pacientes con VAA. Los eventos trombóticos se definieron como aquellos de trombosis arterial y venosa confirmada mediante resonancia magnética, tomografía computarizada, angiografía o ecografía Doppler. Se compararon las variables clínicas y analíticas en el momento del diagnóstico y durante el seguimiento entre los pacientes con y sin eventos trombóticos. Se realizó un análisis con un modelo de riesgos de Cox univariable y multivariable para obtener de forma adecuada el cociente de riesgos instantáneos (hazard ratio [HR]) teniendo en cuenta la duración del seguimiento.
ResultadosLa edad media de los 138 pacientes con VAA fue de 55,1 años, y 44 pacientes eran varones. Se detectó SAF persistente en 18 pacientes con VAA (13,0%). Se observaron eventos trombóticos en 26 pacientes con VAA (18,8%) durante el seguimiento. En un punto de análisis retrospectivo del estudio se observó SAF persistente en el momento del diagnóstico de forma más frecuente en pacientes con eventos trombóticos que en aquellos sin este tipo de eventos. En el análisis con un modelo de riesgos de Cox multivariable, la edad en el momento del diagnóstico (HR: 1,075) y SAF persistente (HR: 2,902), pero no ANCA. Los eventos trombóticos se identificaron de forma más frecuente en los pacientes con SAF persistente en el momento del diagnóstico que en aquellos sin SAF (38,9% frente a 15,8%; riesgo relativo 3,383).
ConclusiónEl SAF persistente en el momento del diagnóstico se asoció de forma significativa a un riesgo de eventos trombóticos durante el seguimiento de la VAA. Se sugiere que los médicos deberían monitorizar de forma exhaustiva el desarrollo de eventos trombóticos durante el seguimiento.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of systemic vasculitides, which are characterised by necrotising vasculitis with few or no deposition of immune complex and by encroaching small sized vessels from capillaries to arteriole and venules.1 ANCA consists of myeloperoxidase (MPO)-ANCA or perinuclear (P)-ANCA and proteinase 3 (PR3)-ANCA or cytoplasmic (C)-ANCA.2 AAV can be categorised as three variants according to histological findings, ANCA positivity and clinical features: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic GPA (EGPA).1,3–5 GPA often exhibits its surrogate clues including upper or lower airway symptoms from sinusitis to cavitary nodule in lungs and granuloma on histology and EGPA is mainly associated with asthma, eosinophilia, migratory lung lesions, neuropathy and eosinophilic degranulation or granuloma on histology.1,3–5 Meanwhile, MPA is related to renal vasculitis and diffuse alveolar haemorrhage, but shows no granuloma or eosinophilic infiltration on histology.1,3
Antiphospholipid syndrome (APS) is characterised by vascular thrombosis ranging from veins to arteries and recurrent spontaneous abortion predominantly between 10 and 34 intrauterine weeks under persistent antiphospholipid antibodies (APLAs) with an interval of 12 weeks or greater.6 APLAs include anti-cardiolipin (aCL) IgM or IgG, anti-beta2glycoprotein 1 (anti-β2GP1) IgM and IgG and lupus anticoagulant (LAC). APS classification can be done when one clinical item or more and one laboratory items or more are fulfilled.6,7 Because persistent APLAs can accelerate atherosclerosis and immune-mediated endothelial inflammation, they may provoke serious cerebrovascular, cardiovascular and peripheral vascular thrombosis.8 Thus, regardless of APS diagnosis, persistent APLAs may increase the risk of arterial and venous thrombotic events in AAV patients. A previous study reported that persistent APLAs, particularly LAC, were positively proportional to vasculitis damages in AAV during follow-up.9 However, so far, there was no report on the association between persistent APLAs and thrombotic events in a considerable number of patients with AAV. Hence, in this study, we investigated whether persistent APLAs at diagnosis are associated with the risk of thrombotic events during follow-up in AAV patients.
ObjectivesIn this study, we investigated whether persistent APLAs at diagnosis are associated with the risk of thrombotic events during follow-up in AAV patients.
Materials and methodsPatientsWe retrospectively reviewed the details of medical records of 138 AAV patients according to the inclusion criteria as follows: (i) patients who were first classified as AAV from October 2000 to September 2017 at Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital; (ii) patients who fulfilled the American College of Rheumatology 1990 criteria for the classification of GPA and EGPA and then reclassified by the algorithm suggested by the European Medicines Agency in 2007, in which authors added the modified contents of the Chapel Hill Consensus Conferences (CHCC) Nomenclature of Vasculitis proposed in 20121,3–5; (iii) patients who had been followed up for 12 weeks or greater after the second results of APLAs10; (iv) patients who had well-documented medical records to assess Birmingham vasculitis activity score (BVAS) and prognostic five factor score (FFS) (2009) at diagnosis11–13; (v) patients who had the results of blood tests for perinuclear (P)-ANCA and cytoplasmic (C)-ANCA or myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA at diagnosis, regardless of positivity2; (vi) patients who had not been classified as definite APS prior to this study; (vii) patients who had no medical history to affect either items of BVAS, ANCA positivity or laboratory results, such as malignancies, haematological disorders, chronic liver diseases and autoimmune diseases other than AAV, which were identified in the 10th revised International Classification Diseases (ICD-10); (viii) patients who had never received drugs for those medical conditions (vi and vii) at diagnosis, which were searched by the Korean Drug Utilisation Review (DUR) system. This study was approved by the institutional Review Board of Severance Hospital (4-2017-0673), and the patient's written informed consent was waived by the approving IRB, as this was a retrospective study.
Clinical data, comorbidities and medicationsWe collected age, gender, smoking history and the follow-up duration. The follow-up duration was defined as the period from diagnosis to the first thrombotic events for patients with thrombotic events and that from diagnosis to the last visit for patients without. We obtained clinical manifestations at diagnosis based on organ-specific detailed items of BVAS,11,12 and calculated it. ANCAs and APLAs at diagnosis were collected. We searched medical conditions regarding comorbidities during follow-up by ICD-10. We also collected medications administered during follow-up by under the Korean DUR system.
Persistent APLAsPersistent APLAs was defined, when any type of APLAs, such as aCL IgM or IgG, anti-β2GP1 IgM and IgG and LAC, is serially detected with an interval of 12 weeks or greater.
Thrombotic eventsThrombotic events were defined as arterial and venous thrombosis confirmed by magnetic resonance imaging, computed tomography, angiography and Doppler ultrasonography. And they were categorised as cardiovascular or peripheral arterial thrombotic events, cerebrovascular thrombotic events and venous thrombotic events.
Statistical analysesAll statistical analyses were conducted using SPSS software (version 23 for windows; IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation, and categorical variables were done as number and the percentage. Significant differences between patients with and without thrombotic events were compared using the chi square and Fisher's exact tests for categorical data and the Mann–Whitney U test for continuous variables. The multivariable Cox hazard model using variables with statistical significance was conducted in univariable Cox hazard model to appropriately hazard ratio (HR). The relative risk (RR) of the presence of persistent APLAs for thrombosis was analysed using contingency tables and the chi square test. P-values less than 0.05 were considered statistically significant.
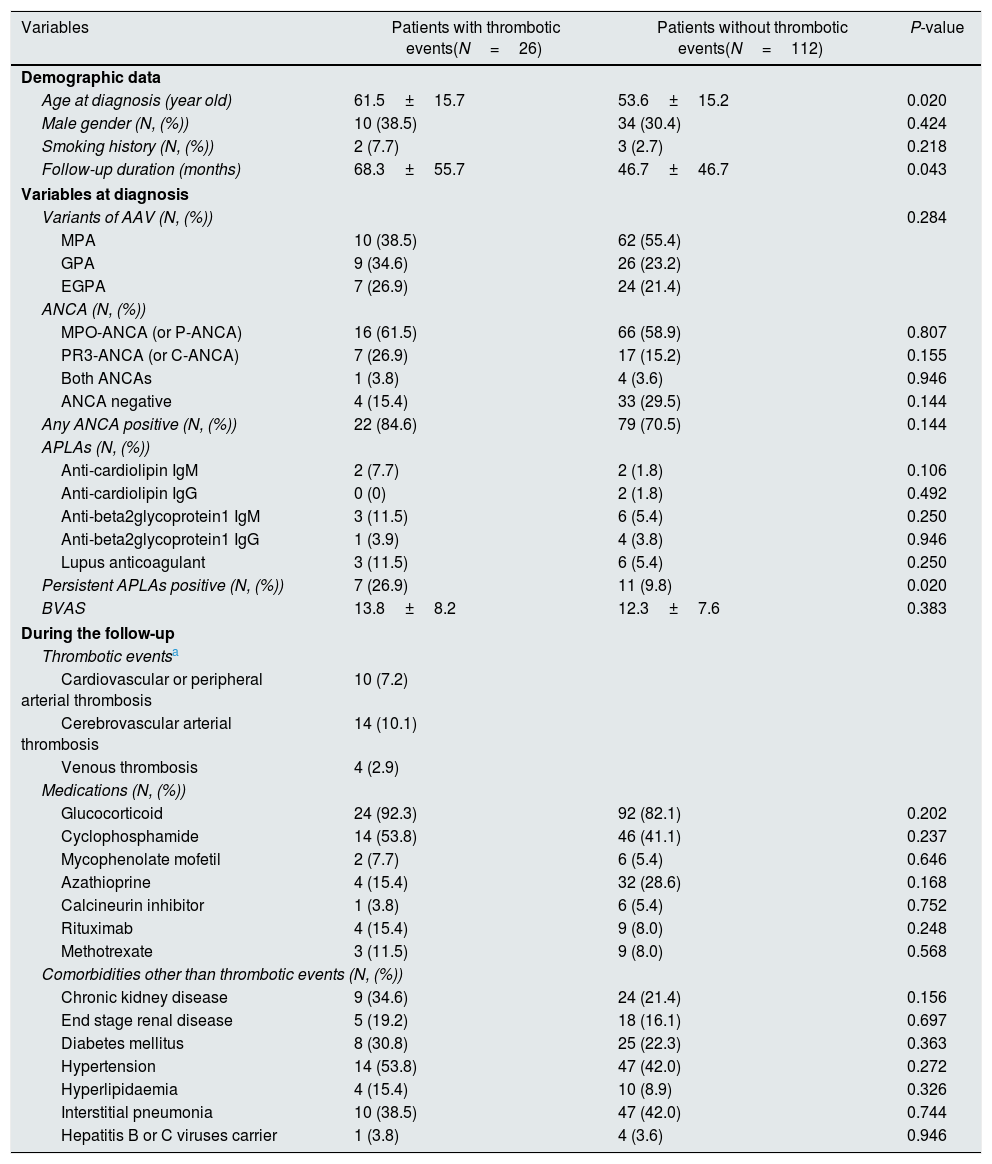
ResultsBaseline characteristics of 138 AAV patientsThe mean age of 138 AAV patients was 55.1 years and 44 were male. The mean follow-up duration was 50.8 months. Seventy-two patients (52.2%) had MPA, 35 patients (25.4) had GPA and 31 patients (22.5%) had EGPA. The initial mean BVAS and FFS (2009) were 12.6 and 1.2. Among comorbidities, hypertension was the most frequently observed. Thrombotic events were observed in 26 patients with AAV (18.8%) during the follow-up (Table 1).
Comparison of variables between AAV patients with and without thrombotic events.
| Variables | Patients with thrombotic events(N=26) | Patients without thrombotic events(N=112) | P-value |
|---|---|---|---|
| Demographic data | |||
| Age at diagnosis (year old) | 61.5±15.7 | 53.6±15.2 | 0.020 |
| Male gender (N, (%)) | 10 (38.5) | 34 (30.4) | 0.424 |
| Smoking history (N, (%)) | 2 (7.7) | 3 (2.7) | 0.218 |
| Follow-up duration (months) | 68.3±55.7 | 46.7±46.7 | 0.043 |
| Variables at diagnosis | |||
| Variants of AAV (N, (%)) | 0.284 | ||
| MPA | 10 (38.5) | 62 (55.4) | |
| GPA | 9 (34.6) | 26 (23.2) | |
| EGPA | 7 (26.9) | 24 (21.4) | |
| ANCA (N, (%)) | |||
| MPO-ANCA (or P-ANCA) | 16 (61.5) | 66 (58.9) | 0.807 |
| PR3-ANCA (or C-ANCA) | 7 (26.9) | 17 (15.2) | 0.155 |
| Both ANCAs | 1 (3.8) | 4 (3.6) | 0.946 |
| ANCA negative | 4 (15.4) | 33 (29.5) | 0.144 |
| Any ANCA positive (N, (%)) | 22 (84.6) | 79 (70.5) | 0.144 |
| APLAs (N, (%)) | |||
| Anti-cardiolipin IgM | 2 (7.7) | 2 (1.8) | 0.106 |
| Anti-cardiolipin IgG | 0 (0) | 2 (1.8) | 0.492 |
| Anti-beta2glycoprotein1 IgM | 3 (11.5) | 6 (5.4) | 0.250 |
| Anti-beta2glycoprotein1 IgG | 1 (3.9) | 4 (3.8) | 0.946 |
| Lupus anticoagulant | 3 (11.5) | 6 (5.4) | 0.250 |
| Persistent APLAs positive (N, (%)) | 7 (26.9) | 11 (9.8) | 0.020 |
| BVAS | 13.8±8.2 | 12.3±7.6 | 0.383 |
| During the follow-up | |||
| Thrombotic eventsa | |||
| Cardiovascular or peripheral arterial thrombosis | 10 (7.2) | ||
| Cerebrovascular arterial thrombosis | 14 (10.1) | ||
| Venous thrombosis | 4 (2.9) | ||
| Medications (N, (%)) | |||
| Glucocorticoid | 24 (92.3) | 92 (82.1) | 0.202 |
| Cyclophosphamide | 14 (53.8) | 46 (41.1) | 0.237 |
| Mycophenolate mofetil | 2 (7.7) | 6 (5.4) | 0.646 |
| Azathioprine | 4 (15.4) | 32 (28.6) | 0.168 |
| Calcineurin inhibitor | 1 (3.8) | 6 (5.4) | 0.752 |
| Rituximab | 4 (15.4) | 9 (8.0) | 0.248 |
| Methotrexate | 3 (11.5) | 9 (8.0) | 0.568 |
| Comorbidities other than thrombotic events (N, (%)) | |||
| Chronic kidney disease | 9 (34.6) | 24 (21.4) | 0.156 |
| End stage renal disease | 5 (19.2) | 18 (16.1) | 0.697 |
| Diabetes mellitus | 8 (30.8) | 25 (22.3) | 0.363 |
| Hypertension | 14 (53.8) | 47 (42.0) | 0.272 |
| Hyperlipidaemia | 4 (15.4) | 10 (8.9) | 0.326 |
| Interstitial pneumonia | 10 (38.5) | 47 (42.0) | 0.744 |
| Hepatitis B or C viruses carrier | 1 (3.8) | 4 (3.6) | 0.946 |
Values are expressed as mean and standard deviation or N (%).
Of 26 patients with thrombotic events, two patients exhibited two thrombotic events.
AAV: antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis; MPA: microscopic polyangiitis; GPA: granulomatosis with polyangiitis; EGPA: eosinophilic granulomatosis with polyangiitis; MPO: myeloperoxidase; P: perinuclear; PR3: proteinase 3; C: cytoplasmic; APLAs: antiphospholipid antibodies; BVAS: Birmingham vasculitis activity score.
When we divided 138 AAV patients according to thrombotic events, twenty-six patients were belonging to thrombotic event group. Of 26 patients with thrombotic lesions, 10 patients (7.2%) exhibited cardiovascular or peripheral arterial thrombosis, 14 patients (10.1%) exhibited cerebrovascular thrombotic lesions and 4 patients (2.9%) had venous thrombosis. Two patients exhibited 2 thrombotic events. Among variables at diagnosis, patients with thrombotic events exhibited the higher mean age and the follow-up duration than those without (61.5 vs. 53.6 years old and 68.3 vs. 46.7 months). There were no significant differences in APLA-types including aCL IgM and IgG, anti-β2GP1 IgM and IgG and LAC. However, when we defined the presence of any type of APLAs as persistent APLAs, persistent APLAs were detected more frequently in patients with thrombotic events than those without (26.9% vs. 9.8%). However, any type of ANCA was detected evenly in the two groups. There were no significant differences in medications and comorbidities between patients with and without thrombotic events (Table 1).
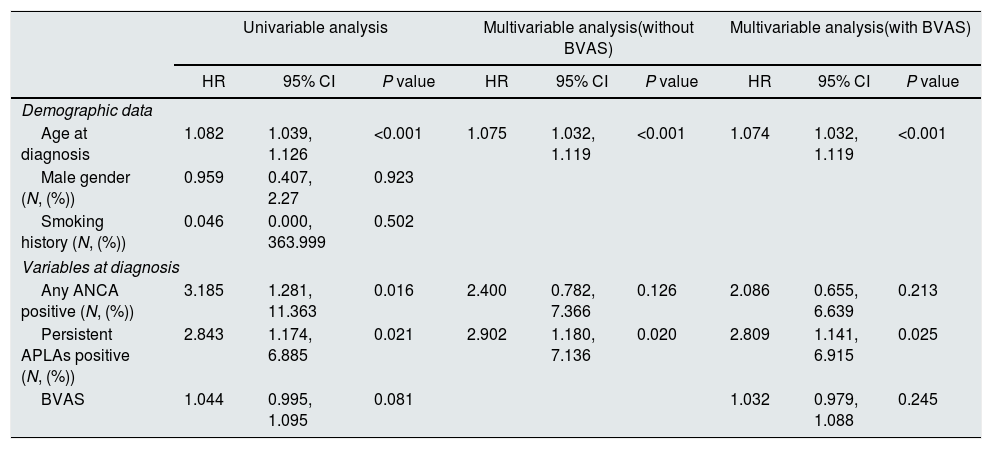
Predictive value of persistent APLAs for thrombotic eventsConsidering the follow-up duration, we performed univariable Cox hazard model analysis with all variables at diagnosis. In the univariable Cox hazard model analysis using variables at diagnosis, age at diagnosis (HR 1.082, P<0.001), any ANCA positive at diagnosis (HR 3.185, P=0.016) and persistent APLAs positive at diagnosis (HR 2.843, P=0.021). BVAS at diagnosis (HR 1.044, P=0.081) tended to be associated with thrombotic event-development, but it was not significant. In the multivariable Cox hazard model analysis using three variables with significance in the univariable analysis, age at diagnosis (HR 1.075, 95% confidence interval [CI] 1.032, 1.119) and persistent APLAs positive at diagnosis (HR 2.902, 95% CI 1.180, 7.136). Any ANCA positive at diagnosis was not associated with thrombotic events during follow-up in AAV patients. In addition, when we included BVAS in the multivariable Cox hazard model analysis, only age at diagnosis (HR 1.074, 95% CI 1.032, 1.119) and persistent APLAs positive at diagnosis (HR 2.809, 95% CI 1.141, 6.915) (Table 2).
Multivariable Cox hazard model of variables at diagnosis for thrombotic events with those with significance in univariable analysis in patients with AAV.
| Univariable analysis | Multivariable analysis(without BVAS) | Multivariable analysis(with BVAS) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Demographic data | |||||||||
| Age at diagnosis | 1.082 | 1.039, 1.126 | <0.001 | 1.075 | 1.032, 1.119 | <0.001 | 1.074 | 1.032, 1.119 | <0.001 |
| Male gender (N, (%)) | 0.959 | 0.407, 2.27 | 0.923 | ||||||
| Smoking history (N, (%)) | 0.046 | 0.000, 363.999 | 0.502 | ||||||
| Variables at diagnosis | |||||||||
| Any ANCA positive (N, (%)) | 3.185 | 1.281, 11.363 | 0.016 | 2.400 | 0.782, 7.366 | 0.126 | 2.086 | 0.655, 6.639 | 0.213 |
| Persistent APLAs positive (N, (%)) | 2.843 | 1.174, 6.885 | 0.021 | 2.902 | 1.180, 7.136 | 0.020 | 2.809 | 1.141, 6.915 | 0.025 |
| BVAS | 1.044 | 0.995, 1.095 | 0.081 | 1.032 | 0.979, 1.088 | 0.245 | |||
AAV: antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis; BVAS: Birmingham vasculitis activity score; HR: hazard ratio; CI: confidence interval; APLAs: antiphospholipid antibodies.
When we divided AAV patients into two groups according to the presence of persistent APLAs, 18 of 138 patients (13.0%) had persistent APLAs at diagnosis. Thrombotic events were identified more frequently in patients with persistent APLAs at diagnosis than those without (38.9% vs. 15.8%, P=0.020). Furthermore, patients with persistent APLAs at diagnosis exhibited a significantly high RR of thrombotic events compared to those without (RR 3.383, 95% CI 1.164, 9.831) (Fig. 1).
Relative risk of persistent APLAs for thrombosis. Thrombotic events were identified more frequently in patients with persistent APLAs at diagnosis than those without. Furthermore, patients with persistent APLAs at diagnosis exhibited a significantly high RR of thrombotic events compared to those without. APLAs: antiphospholipid antibodies; RR: relative risk.
In this study, we investigated whether persistent APLAs at diagnosis are associated with the risk of thrombotic events during follow-up. We demonstrated that, at the time of a retrospective study point, persistent APLAs at diagnosis were observed more frequently in patients with thrombotic events than those without. Furthermore, we drew the conclusion that persistent APLAs at diagnosis were associated with thrombotic events during follow-up, comparable to age at diagnosis. Male gender, hypertension, diabetes mellitus, dyslipidaemia and chronic renal diseases, which are conventional risk factors for cardiovascular or cerebrovascular diseases in general population,14 were not related to thrombotic events in this study population.
Persistent APLAs can provoke thrombogenic state by driving endothelial cells to express adhesion molecules and up-regulate tissue factor production along with monocytes and enforcing platelets to produce glycoprotein 2b-3a and thromboxane A2 production.8 Persistent APLAs can also advance thrombogenic state to thrombosis state by activating complement-cascades system and augmenting the interaction with coagulation-regulatory proteins.8 On the other hands, the severity of AAV is considered associated with platelet activation markers and coagulation or fibrinolysis indices.15,16 Thus, theoretically, all kinds of APLAs can increase the risk of thrombotic complication in AAV patients at a high rate. In this study, we elucidated that persistent APLAs at diagnosis did affect thrombotic events during follow-up in AAV patients. Thus, we suggest that physicians should consider the need for preventive anti-aggregation or anticoagulation therapies in AAV patients with the concomitant presence of APLAs at diagnosis or during follow-up.
Venous thrombosis occurring in GPA patients is the most representative among vascular thrombotic events in AAV patients. A previous prospective and observational cohort study reported the incidence of venous thrombosis as 7.0 per 100 person-years in 180 GPA patients.17 In addition, a retrospective French cohort study provided a results of similar venous thrombosis incidences in MPA and EGPA patients to that in GPA patients.18 In our study, venous thrombosis was observed in one of 72 MPA patients (1.4%), 2 of 35 GPA patients (5.7%) and 1 of 31 EGPA patients (3.2%), but there was no statistical significance. Meanwhile, the increased risk of arterial thrombosis in MPA, GPA and EGPA has been reported, and furthermore arterial thrombotic events were often found in the earlier phase of AAV.19,20 In our study, ischaemic heart disease was observed in 5 of 72 in MPA patients (6.9%), 3 of 35 GPA patients (8.6%) and 2 of 31 EGPA patients (6.5%). Cerebrovascular accident was done 4 in MPA patients (5.6%), 5 in GPA patients (14.3%) and 5 in EGPA patients (16.1%) without statistical significance. On the other hands, the mean follow-up period from diagnosis to thrombotic events in our study-patients with thrombotic events was 68.3 months, which was much longer than that in those without. We concluded that AAV type and the short follow-up period did not affect the incidence of thrombotic events in our study.
Our study has an advantage that we first demonstrated that persistent APLAs at diagnosis were associated with thrombotic events during follow-up in AAV patients. Our study also has several issues: First, because this study was designed as a retrospective study, we could not serially measure APLAs during follow-up. Second, due to the small number of patients with APLAs, we could not identify the association of subtype of APLAs with thrombotic events in AAV patients. Third, given that the prevalence of APLAs in general population might differ according to the geographic or ethnic backgrounds, it must be better to provide the information on them in the general population in Korea, in order to clarify the clinical implication of APLAs in AAV patients. However, we could not compare the prevalence and the clinical implication of APLAs between AAV patients and healthy population due to lack of data on the general population in Korea. Future prospective studies with larger patients and serial measurement of APLAs titres will provide more dynamic and convincing information on the influence of persistent APLAs on thrombotic events in patients with AAV.
ConclusionsPersistent APLAs at diagnosis are significantly associated with the risk of thrombotic events during follow-up of AAV. We suggest that physicians should pay more attention to AAV patients having persistent APLAs at diagnosis, encourage them to visit the clinic more often and closely monitor the development of thrombotic events during follow-up.
FundingThis study was supported by a faculty research grant of Yonsei University College of Medicine (6-2016-0145).
Conflict of interestThe authors declare no competing interests.