Matrix metalloproteinases (MMPs) are involved in deleterious tissue remodelling associated with target organ damage in renal disease. The aim of this study was to study the association between renal dysfunction and activity of the inflammatory metalloproteinase MMP-9 in hypertensive patients with mild-moderate chronic kidney disease (CKD).
Material and methodsPlasmatic active MMP-9, total MMP-9, tissue inhibitor of MMP-9 (TIMP-1), MMP-9/TIMP-1 ratio and MMP-9-TIMP-1 interaction were analysed in 37 hypertensive patients distributed by estimated glomerular filtration rate (eGFR) in 3 groups: >90, 90–60 and 60–30mL/min/1.73m2.
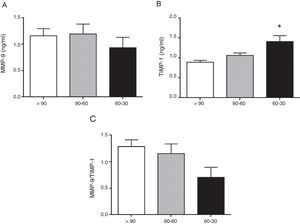
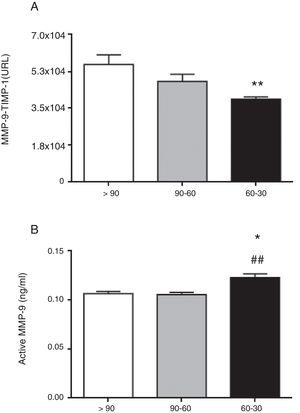
ResultsTotal MMP-9 was not different as eGFR declines. TIMP-1 was significantly increased in hypertensive patients with eGFR 60–30mL/min/1.73m2 (p<0.01 vs. >90mL/min/1.73m2). This relates to the significant decrease in the interaction between MMP-9-TIMP-1 observed in patients with eGFR 60–30mL/min/1.73m2 (p<0.01 vs. >90mL/min/1.73m2). Despite the systemic elevation of TIMP-1, active MMP-9 was significantly increased in hypertensive patients with eGFR 60–30mL/min/1.73m2 (p<0.05 and p<0.01 vs. >90 and 90–60mL/min/1.73m2, respectively). TIMP-1, active MMP-9 and MMP-9-TIMP-1 interaction significantly correlate with the decline in renal function, which was not observed with total MMP-9.
ConclusionsThe progression of CKD, even in stages where the decline of renal function is still moderate, is associated with an increase in MMP-9 activity, which could be considered as a potential therapeutic target.
Las enzimas metaloproteinasas de matriz (MMP) están involucradas en el remodelado tisular deletéreo asociado al daño de órganos diana de la enfermedad renal. El objetivo de este estudio fue explorar la asociación entre la caída de la función renal y la actividad sistémica de la metaloproteinasa inflamatoria MMP-9 en el paciente hipertenso con enfermedad renal crónica (ERC) leve-moderada.
Material y métodosSe analizaron los niveles plasmáticos de MMP-9 activa, MMP-9 total, su inhibidor tisular (TIMP-1), el cociente MMP-9/TIMP-1 y la interacción entre MMP-9 y TIMP-1 en 37 pacientes hipertensos distribuidos según su tasa de filtración glomerular estimada (TFGe) en 3 grupos:>90, 90–60 y 60–30mL/min/1,73m2.
ResultadosLa MMP-9 total no fue diferente con respecto a la disminución en la TFGe. TIMP-1 estaba significativamente incrementado en los pacientes hipertensos con TFGe entre 60–30mL/min/1,73m2 (p<0,01 versus>90mL/min/1,73m2). Estos resultados fueron apoyados por la disminución significativa de la interacción MMP-9-TIMP-1 observada en los pacientes con TFGe entre 60–30mL/min/1,73m2 (p<0,01 versus>90mL/min/1,73m2). A pesar de la elevación sistémica de TIMP-1 encontramos un incremento significativo de MMP-9 activa en los pacientes hipertensos con TFGe entre 60–30mL/min/1,73m2 (p<0,05 y p<0,01 versus>90 y 90–60mL/min/1,73m2, respectivamente). Los niveles de TIMP-1, MMP-9 activa e interacción proteica MMP-9-TIMP-1 correlacionaron significativamente con el deterioro de la función renal, lo cual no se observó para la MMP-9 total.
ConclusionesLa progresión de la ERC, incluso en estadios donde la caída de la función renal es aún moderada, se asocia con un aumento específico de la actividad MMP-9, lo cual podría considerarse como una potencial diana terapéutica.
In recent years, it has been shown that in patients with hypertension persist a high residual cardiovascular (CV) and renal risk.1,2 Studies performed by different research groups3–6 have shown that the classic renal marker of target organ damage, albuminuria, can occur in patients with essential hypertension even with a prolonged antihypertensive treatment with blockade of the renin angiotensin system. For example, a decrease in estimated glomerular filtration rate (eGFR) and the presence of albuminuria was recently shown to be significantly associated with a substantial increase in nocturnal systolic blood pressure (BP) (probably one of the phenotypes associated with higher CV risk), especially in moderate/severe stages (stages 3–5) of chronic kidney disease (CKD).6 All these data indicate that, despite adequate pharmacological treatment and the introduction of treatment when necessary to maintain adequate BP values, renal risk persists in patients with hypertension.
The histological alterations in CKD associated with hypertension are mainly glomerulosclerosis, interstitial fibrosis and arteriosclerosis,2 all easily detectable in advanced stages of CKD. However, with specific biomarkers detected systemically, we would be able to detect maladaptive remodelling processes and fibrosis associated with progressive abnormalities of the cardio-renal axis in earlier stages of kidney disease. The matrix metalloproteinase (MMP) enzymes and their tissue inhibitors (TIMP) have a very relevant role as they are directly involved in the remodelling of the extracellular matrix (ECM), a crucial mechanism for the development and progression of CKD. MMP and TIMP also function as biomarkers and changes in their levels or concentrations and/or their systemic activity are associated with inflammatory processes and deleterious remodelling in the cardio-renal axis.7–12 Specifically, changes in the activity of the inducible MMP-9 isoform lead to structural alterations in the renal tubule and glomerulus, particularly in advanced stages of CKD when patients develop severe renal fibrosis.13 Far less is known about the involvement of this inducible metalloproteinase MMP-9 in earlier stages of CKD, however, especially in the context of hypertension. Our aim, therefore, was to make a comparative study of the total circulating levels of MMP-9 and its tissue inhibitor TIMP-1 and the degree of interaction between the two proteins and the amount of active MMP-9 in patients with hypertension and mild/moderate decrease in renal function.
MethodsStudy populationIn this study, we included 37 patients aged older than 18 with essential primary hypertension from the Hypertension Unit of the Hospital Universitario 12 de Octubre Nephrology Department in Madrid. Patients were considered to have essential hypertension if they had systolic/diastolic BP≥140/90mmHg measured in the clinic following the procedure of the European guidelines for the management of arterial hypertension.14,15 Patients with diabetes or primary hyperaldosteronism were excluded from the study. The eGFR was calculated using the CKD-EPI formula,16 and patients were divided into three groups as follows: (1) >90mL/min/1.73m2; (2) from 90 to 60mL/min/1.73m2; and (3) from 60 to 30mL/min/1.73m2.2,15 BP was measured in the clinic using (Omron semi-automatic sphygmomanometer) and by ambulatory BP monitoring (Spacelabs Healthcare monitor ABPM 90207/17). All patients signed an informed consent form before inclusion in the study. The study was approved by the Hospital Universitario 12 de Octubre Ethics Committee and performed in accordance with the principles of the Declaration of Helsinki.
Assay of total matrix metalloproteinase-9 enzyme concentration, its tissue inhibitor-1 and active matrix metalloproteinase-9 enzyme by enzyme-linked immunosorbent assayThe plasma concentration of total MMP-9 and TIMP-1 was determined using commercial kits of enzyme-linked immunosorbent assay (ELISA) following the manufacturer's specifications (Quantikine®, R&D Systems). According to the manufacturer's specifications, the ELISA for total human MMP-9 (DMP900) has a sensitivity of 0.156ng/ml with an intra-assay coefficient of variation of 2.3% and an inter-assay coefficient of variation of 7.5%. Similarly, the ELISA for total human TIMP-1 (DTM100) has a sensitivity of 0.08ng/ml and a coefficient of variation of 4.4% and 4.2% intra- and inter-assay, respectively. The estimation of the interaction between MMP-9 and TIMP-1 was determined indirectly by calculating the MMP-9/TIMP-1 ratio. The quantity of active MMP-9 in the patients’ plasma samples was determined using a specific commercial kit, with a sensitivity of 0.005ng/ml following the manufacturer's (QuickZyme Biosciences) specifications.
Specific analysis of the interaction total matrix metalloproteinase-9 enzyme/tissue inhibitor-1 interaction by immunoassay using AlphaLISA technologyAn immunoassay using AlphaLISA (PerkinElmer) technology was used for this purpose; it was designed and developed by our research group to specifically measure the protein interaction between MMP-9 and TIMP-1 in plasma samples (for more detail see the recently published protocol).17
Statistical analysisContinuous variables were compared using Student's t-test or the unidirectional ANOVA with the Newman–Keuls test, and non-parametric variables were compared with the Kruskal–Wallis test. Categorical variables were compared using Fisher's exact test. Correlations were calculated with the Pearson coefficient of correlation. Data analysis was performed using GraphPad Prism 6 software. The data are presented as mean±standard error of the mean, and statistical significance was considered for p<0.05.
ResultsDemographic and biochemical parametersTable 1 shows the demographic, clinical and biochemical characteristics of the patients with essential hypertension included in this study, divided according to the stage of CKD through eGFR into: (1) eGFR>90ml/1.73min/m2; (2) eGFR 90–60ml/1.73min/m2; and (3) eGFR 60–30ml/1.73min/m2. There were no significant differences between one group of patients and another in BMI, total cholesterol, HDL, LDL or triglyceride levels. Table 1 also shows the BP data measured both in the clinic and by 24h ambulatory BP monitoring; there were no differences between the three groups of study patients in these variables. There were no significant differences in the frequency or the type of antihypertensive (ACE inhibitor or ARB) or lipid-lowering (statins) medication. The significant differences found were in age (p<0.001), serum creatinine levels (p<0.001) and albumin/creatinine ratio (p=0.002).
Clinical and biochemical characteristics of the patients included in the study.
| >90 (n=12) | 90–60 (n=17) | 60–30 (n=8) | p-Value | |
|---|---|---|---|---|
| Male (%, n) | 42 (5) | 59 (10) | 88 (7) | 0.123 |
| Age (years) | 59.3±9.2 | 62.7±9.0 | 74.6±4.9*,** | <0.001 |
| BMI (kg/m2) | 29.0±4.4 | 28.4±2.4 | 29.7±4.2 | 0.676 |
| eGFR (mL/min/1.73m2) | 97.6±4.6 | 78.1±8.7*** | 45.0±11.1*,**** | <0.001 |
| Serum creatinine (mg/dl) | 0.708±0.122 | 0.934±0.128*** | 1.514±0.299*,***** | <0.001 |
| Albumin/creatinine (mg/g) | 31.4 (10–115) | 12.8 (2–41) | 392.1 (64–1641)** | 0.002 |
| Clinic SBP (mmHg) | 129.7±15.7 | 136.2±16.2 | 141.5±30.2 | 0.417 |
| Clinic DBP (mmHg) | 81.8±8.8 | 85.6±9.4 | 85.8±16.9 | 0.614 |
| 24-h SBP (mmHg) | 125.9±14.7 | 124.1±10.3 | 123.0±10.6 | 0.932 |
| 24-h DBP (mmHg) | 79.2±10.6 | 75.7±9.9 | 73.6±9.9 | 0.463 |
| Cholesterol (mg/dl) | 180.4±22.9 | 190.5±33.5 | 169.0±25.2 | 0.222 |
| HDL (mg/dl) | 57.8±16.8 | 50.2±12.0 | 48.6±5.8 | 0.207 |
| LDL (mg/dl) | 101.5±23.9 | 115.8±28.8 | 96.8±19.9 | 0.267 |
| Triglycerides (mg/dl) | 105.8±47.2 | 130.0±62.5 | 117.5±39.0 | 0.491 |
| Statins (%, n) | 92 (11) | 65 (11) | 75 (6) | 0.145 |
| ACE inhib. (%, n) | 25 (3) | 18 (3) | 13 (1) | 0.770 |
| ARB (%, n) | 58 (7) | 76 (13) | 75 (6) | 0.909 |
The patients have been divided according to their eGFR (mL/min/1.73m2). ACE inhib.: angiotensin-converting enzyme inhibitors; ARB: angiotensin-receptor blockers; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HDL: high-density lipoproteins; LDL: low-density lipoproteins; n: number of patients; SBP: systolic blood pressure.
The circulating concentration of total MMP-9 did not vary as CKD progressed in the study patients with hypertension (Fig. 1A). In contrast, the total circulating concentration of its tissue inhibitor TIMP-1 was significantly higher in patients with hypertension who had an eGFR in the range of 60–30mL/min/1.73m2 compared to those who had an eGFR>90mL/min/1.73m2 (p<0.01 [Fig. 1B]). There was no difference among the three groups of patients with hypertension in the ratio between the total levels of MMP-9 and TIMP-1 detected (MMP-9/TIMP-1), widely used in preclinical research as an indirect estimator of MMP-9 activity (Fig. 1C). Moreover, supporting these results, we found a significant decrease in the actual protein interaction between MMP-9 and TIMP-1 systemically, measured by AlphaLISA technology, in patients with hypertension and eGFR in the range of 60–30mL/min/1.73m2 compared to those with an eGFR>90mL/min/1.73m2 (p<0.01 [Fig. 2A]). When the circulating levels of the active MMP-9 isoform were estimated specifically, they were found to be significantly higher in the patients with hypertension and eGFR in the range of 60–30mL/min/1.73m2 (Fig. 2B), than in those with an eGFR in the range of 90–60mL/min/1.73m2 (p<0.01) or those with an eGFR>90mL/min/1.73m2 (p<0.05).
Protein interaction between MMP-9 and its tissue inhibitor TIMP-1 analysed by AlphaLISA (A) and active MMP-9 (B) in the plasma samples of hypertensive patients with eGFR>90, 90–60 and 60–30mL/min/1.73m2. *p<0.05 vs. patients with eGFR>90mL/min/1.73m2. **p<0.01 vs. patients with eGFR90-60 mL/min/17.3m2.
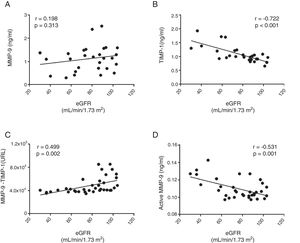
No correlation was found between the total circulating levels of MMP-9 and the decrease in renal function measured by eGFR (r=0.198, p=0.313 panel [Fig. 3A]). In fact, there was a significant negative correlation between eGFR and the total concentration of TIMP-1 (r=−0.722, p>0.001 [Fig. 3B]). A significant positive correlation was found between eGFR and the protein-protein interaction between MMP-9 and TIMP-1 (r=0.499, p=0.002 [Fig. 3C]), such that, as eGFR decreased, there was less interaction between MMP-9 and its inhibitor TIMP-1. In fact, there was a significant negative correlation between the concentration of the active isoform MMP-9 and eGFR (r=−0.531, p>0.001 [Fig. 3D]). Therefore, as renal function decreased, the quantity of TIMP-1 rose and, although it is a physiological inhibitor of MMP-9, the two did not interact, resulting in an increase in the activity of proinflammatory MMP-9. After adjusting all these remodelling parameters for the gender and/or age of the patients, only the total TIMP-1 or active MMP-9 parameters maintained these significant correlations (Table 2).
Partial correlations between eGFR and study variables adjusted for gender and/or age.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Total MMP-9 | r=0.123; p=0.541 | r=0.135; p=0.503 | r=0.007; p=0.974 |
| Total TIMP-1 | r=−0.764; p<0.001 | r=−0.468; p=0.014 | r=−0.545; p=0.004 |
| MMP-9/TIMP-1 | r=0.397; p=0.040 | r=0.255; p=0.199 | r=0.129; p=0.529 |
| MMP-9-TIMP-1 interaction | r=0.476; p=0.003 | r=0.218; p=0.202 | r=0.143; p=0.411 |
| Active MMP-9 | r=−0.514; p=0.002 | r=−0.471; p=0.006 | r=−0.441; p=0.012 |
Model 1: adjusted for gender; model 2: adjusted for age; model 3: adjusted for gender and age.
In this study we have analysed the relationship between a mild/moderate decrease in renal function and the circulating levels of MMP-9 and its inhibitor TIMP-1 in pharmacologically controlled patients with essential hypertension. The results we obtained show that MMP-9 activity (Fig. 2B), but not its total systemic concentration (Fig. 1A), increases as renal function decreases (Fig. 3C and Table 2), with that activity being significantly greater in patients with hypertension and eGFR in the range 60–30mL/min/1.73m2, than in earlier stages with eGFR 90–60 or >90mL/min/1.73m2 (Fig. 2B). Surprisingly, the increased activity of MMP-9 is accompanied by a systemic increase in the concentration of its inhibitor TIMP-1 as renal function decreases (Fig. 3B), with the total concentration of TIMP-1 being significantly higher in patients with hypertension and eGFR in the range of 60–30mL/min/1.73m2, than in those with eGFR>90mL/min/1.73m2 (Fig. 1B). Despite having a higher total circulating concentration of TIMP-1 as renal function decreased, we found a reduction in the protein-protein interaction between MMP-9 and TIMP-1 (Fig. 2B), with significantly less interaction in patients with hypertension and eGFR in the range of 60–30mL/min/1.73m2, than in those with eGFR>90mL/min/1.73m2 (Fig. 2A). These data indicate that although patients with eGFR in the range of 60–30mL/min/1.73m2 have more circulating TIMP-1, it is not exerting its inhibitory capacity on MMP-9, as they are not interacting, meaning that MMP-9 is in fact more free of its inhibitor TIMP-1, and also therefore more active, as the CKD progresses.
MMP enzymes are an extensive family of endopeptidases capable of controlling the synthesis and degradation of the components forming the ECM, thus regulating the process of remodelling and fibrosis of different target organs, both in CV and renal disease.18–20 The MMP-9 isoform in particular is a gelatinase enzyme activated primarily in response to inflammatory processes, resulting in the degradation of different components of the ECM which are its substrates, essentially type IV collagen and elastin.21 Changes in the components of the ECM play a role in the progression of CKD. Advanced stages of CKD are characterised by a severe loss of renal function, often accompanied by structural alterations such as the presence of renal interstitial fibrosis and glomerulosclerosis,22 both easily detectable with histological and imaging tests. Associated with this structural damage is a differential pattern of expression and/or activity of biomarkers involved in remodelling, such as MMP-9. At a glomerular level, the infiltration of inflammatory cells, the release of pro-inflammatory cytokines such as tumour necrosis factor alpha (TNF-α) and profibrotic cytokines such as transforming growth factor-beta (TGF-β), as well as the release of reactive oxygen species, increase MMP-9 synthesis and activity, initially as a compensatory mechanism to degrade excess collagen synthesis and so prevent the development of renal fibrosis.13 However, as the CKD progresses, MMP-9 activity begins to wane. This further aggravates the accumulation of components in the ECM, and also as a consequence the renal fibrosis, which by then is already unlikely to be reversible. However, what about the involvement of MMP-9 in earlier stages of CKD before the renal dysfunction is severe? At what stage of the development of CKD does MMP-9 start to play a role? Could MMP-9 serve as an early biomarker of remodelling before renal fibrosis is detected? We found in this study that MMP-9 was significantly more active in patients with controlled hypertension who had eGFR in the range of 60–30mL/min/1.73m2, compared to the earlier stages (90–60 and >90mL/min/1.73m2). It is quite striking that we found no differences in the total circulating expression of MMP-9 but we did find differences in TIMP-1, which would suggest that if we estimated MMP-9 activity indirectly using the MMP-9/TIMP-1 ratio, it would be no different, and MMP-9 would not therefore be active in patients with an eGFR in the range of 60–30mL/min/1.73m2. Surprisingly, by adequately measuring MMP-9 activity and the actual interaction between MMP-9 and TIMP-1 with a new assay developed by our research group8,17which uses AlphaLISA® technology, we found that hypertensive patients with eGFR between 60 and 30mL/min/1.73m2 have significantly more active MMP-9. These results suggest that the role of MMP-9 is probably underestimated in many pathological situations, as in the vast majority of preclinical research studies, only its total systemic concentration is determined and MMP-9 activity is not taken into account.17 More MMP-9 activity in relatively early stages of CKD could have significant physiological repercussions beyond being a compensatory mechanism to avoid the accumulation of collagen deposits and, subsequently, fibrosis. A significant increase in circulating and renal MMP-9 activity has been found in patients who develop albuminuria and in experimental models of spontaneous development of albuminuria in Munich Wistar Frömter rats, related to the strong component of oxidative stress associated with albuminuria.23 At the endogenous level, MMP-9 is under the control of its tissue inhibitor TIMP-1, which can be an oxidative target in situations of systemic increase in oxidative stress.24 Recent studies have shown that the development of albuminuria resistant to the chronic inhibition of the renin angiotensin system is accompanied by a systemic increase in oxidised TIMP-1 (oxyTIMP-1), which is unable to bind with its MMP-9 target, leaving the MMP-9 free and allowing it to remain activated.8 Both conditions, increased oxidative stress and activation of MMP-9, could have a direct impact on the glomerular filtration barrier that can lead to the development of albuminuria in early stages of CKD. This is because the reactive oxygen species are capable of degrading the glycocalyx that covers the fenestrated glomerular endothelium of the capillaries of the glomerulus, resulting in the endothelium losing its strong negative charge, and thus preventing the repulsion of charges with the albumin.25 At the same time, the oxidative stress component stimulates the activation of MMP-9 which, in the kidneys, will act mainly on the glomerular basement membrane (GBM), where its substrate is predominantly type iv collagen. This means that because the barrier is then structurally damaged, both in its endothelial part and in the GBM, the albumin can be filtered more easily from the bloodstream into the urine.
This study has the following limitations: (1) it was a purely descriptive study; and (2) the small number of patients included. For these reasons, it is absolutely essential to have prospective studies with a significantly larger number of patients to corroborate these findings and establish the causality of increased MMP-9 activity in the development of kidney disease.
In conclusion, in this study we demonstrated that there is a significant association between renal dysfunction and specific increase in MMP-9 activity, even in stages where the decline in renal function is still moderate. We have also shown that the role of MMP-9 must not be underestimated, especially in early stages of CKD. Beyond merely measuring circulating concentrations, MMP-9 needs to be properly analysed using biochemical and molecular techniques in order to identify what it actually does.
FundingGRH is a Miguel Servet type I researcher for the ISCIII (Instituto de Salud Carlos III [Carlos III Health Institute]) (CP15/00129). The research activities of GRH, GAL, MGB and LMR are funded by the ISCIII (PI11/0243, PI13/01746, PI14/01841, PIE13/00045, PI17/01193, PI17/01093). This study has been funded primarily by the SENEFRO (Sociedad Red de Investigación Renal Española de Nefrología [Spanish Society of Nephrology]) Foundation, and partially by the Sociedad Española de Cardiología [Spanish Society of Cardiology] and the Íñigo Álvarez de Toledo Foundation. The authors Gloria Álvarez-Llamas and Julián Segura are researchers belonging to the ISCIII RETICS/REDINREN (Redes Temáticas de Investigación Cooperativa en Salud/Red de Investigación Renal [Thematic Networks of Cooperative Research in Health/Renal Research Network]).
Conflicts of interestThe authors of this manuscript have no conflicts of interest to declare.
Please cite this article as: Rodríguez-Sánchez E, Navarro-García JA, Aceves-Ripoll J, Álvarez-Llamas G, Segura J, Barderas MG, et al. Asociación entre disminución de la función renal y actividad metaloproteinasa-9 en el paciente hipertenso. Nefrologia. 2019;39:184–191.