Fabry disease (FD) is a hereditary disorder caused by a deficiency of α-galactosidase A enzyme activity. The transmission of the disorder is linked to the X chromosome.
ObjectivesThe objectives of the study were: 1. To quantify the presence of podocytes in pediatric patients with FD and compare them with the value of the measured podocyturia in healthy controls. 2. To determine whether a greater podocyturia is related to the onset of pathological albuminuria in patients with FD. 3. To determine the risk factors associated with pathological albuminuria.
MethodsWe performed an analytical, observational study of Fabry and control subjects, which were separated into 2 groups in accordance with the absence of the disease (control group) or the presence of the disease (Fabry group).
ResultsWe studied 31 patients, 11 with FD and 20 controls, with a mean age of 11.6 years.
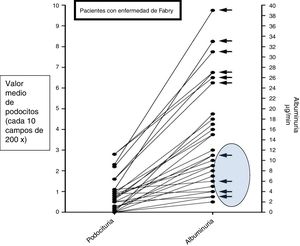
The difference between the mean time elapsed from the diagnosis of FD to the measurement of podocyturia (40 months) and the onset of pathological albuminuria (34 months) was not significant (p=0.09). Podocytes were identified by staining for the presence of synaptopodin and the mean quantitative differences between both podocyturias were statistically significant (p=0.001). Albuminuria was physiological in 4 of the patients with FD and the relative risk to develop pathological albuminuria according to podocyturia was 1.1 in the control group and 3.9 in the Fabry group, with a coefficient of correlation between podocyturia and albuminuria in the Fabry group of 0.8354. Finally, the 2 risk factors associated with the development of pathological albuminuria were podocyturia (OR: 14) and being aged over 10 years (OR: 18). We found no significant risk with regard to glomerular filtrate renal (GFR) (OR: 0.5) or gender (OR: 1.3). The mean GFR remained within normal values.
ConclusionThe detection of podocyturia in pediatric patients with FD could be used as an early marker of renal damage, preceding and proportional to the occurrence of pathological albuminuria.
La enfermedad de Fabry (EF) es un trastorno hereditario causado por una deficiencia de la actividad de la enzima α-galactosidasa A, cuya transmisión está relacionada con el cromosoma X.
ObjetivosLos objetivos del estudio fueron: 1. Cuantificar la presencia de podocitos en pacientes pediátricos con EF y compararla con el valor de la podocituria medida en controles sanos. 2. Determinar en pacientes con EF si una mayor podocituria está relacionada con la albuminuria patológica. 3. Determinar los factores de riesgo asociados con la albuminuria patológica.
MétodosImplementamos un estudio analítico observacional de casos y controles, separados en 2 grupos de acuerdo con la ausencia de enfermedad (grupo control) o con la presencia de enfermedad (grupo Fabry).
ResultadosEstudiamos a 31 pacientes, 11 con EF y 20 controles, con una media de edad de 11,6 años. La diferencia entre el tiempo medio transcurrido desde el diagnóstico de EF hasta la medición de la podocituria (40 meses) y la aparición de la albuminuria patológica (34 meses) no fue significativa (p: 0,09). Los podocitos se identificaron mediante tinción para sinaptopodina y las diferencias medias cuantitativas entre ambas podociturias fueron estadísticamente significativas (p: 0,001). La albuminuria fue fisiológica en 4 de las pacientes Fabry y el riesgo relativo para desarrollar albuminuria patológica de acuerdo con la podocituria fue en el grupo control 1,1 y en el grupo Fabry 3,9, con un coeficiente de correlación entre la podocituria y la albuminuria en el grupo Fabry de 0,8354. Finalmente los 2 factores de riesgo asociados al desarrollo de albuminuria patológica fueron la podocituria (OR 14) y la edad mayor a 10 años (OR 18). No encontramos riesgo significativo ni en el filtrado glomerular (FG) (OR 0,5), ni en el género (OR 1,3). El FG medio se mantuvo dentro de valores normales.
ConclusiónLa detección de podocituria en pacientes pediátricos con EF podría utilizarse como un marcador temprano de daño renal previo y relacionado con la albuminuria patológica.
Fabry disease (FD) is a hereditary disorder caused by the reduced activity of the enzyme α-galactosidase A. It is transmitted linked to the X chromosome and affects more severely to men than women.
These patients fully develop the disease around the fifth decade of life, however, its onset occurs during pediatric age, with specific symptoms such as acroparesthesia, angiokeratomas, corneal opacity and hypo- or anhidrosis.1
In the kidney, the progressive accumulation of glycosphingolipids causes functional alterations of podocytes, endothelial cells, smooth muscle and tubular cells. The main manifestations of this nephropathy are proteinuria with progressive deterioration of renal function.2
The treatment is based on enzyme replacement therapy and general treatment directed to reduce the progression of chronic kidney disease.3
Despite knowing the natural history of the disease and, specifically, the anatomopathological changes in Fabry's nephropathy, the initial mechanism inducing pathological albuminuria and the consequent evolution toward chronic kidney disease are not yet clear.
Although the glomerular basement membrane was always considered the main determinant of the filtration process, it is now evident that it is the visceral epithelium of the Bowman capsule that defines the characteristics of the ultrafiltrate.4 Indeed, the podocyte has a complex structure, formed by a body and its prolongations or foot processes, which are in direct contact with the basement membrane of the glomerular capillary; the space between 2 foot processes constitutes the structure known as filtration diaphragm, with pores from 4 to 14nm.3
Assuming that the detachment of podocytes from the glomerular basement membrane and its appearance in the urine (podocyturia) is a natural process that is increased in the presence of renal damage (for example, the presence of Gb3 deposits in the architecture of the podocyte) then we should accept the presence of minimal podocyturia in healthy individuals and a significant increase would represent an early finding of glomerular disease,4 which leads to the release of podocytes, with their consequent appearance in urine.5 Therefore, the identification of urinary podocytes potentially constitutes a biomarker that precedes the appearance of pathological albuminuria, both in primary and secondary glomerulopathies.6,7
The primary objective of our work was to analyze the urine of pediatric patients with FD, to quantify the presence of podocytes, comparing it with the value of podocyturia measured in healthy children. The secondary objectives were to determine in pediatric patients with FD if a greater podocyturia is related to the appearance of pathological albuminuria and what are potential risk factors that may be involved.
MethodsStudy designThis is observational analytical study of cases and controls, with an unequal distribution of samples (A: 2, B: 1) in 2 groups according to the absence of disease (control group: healthy controls) or with the presence of disease (group Fabry: patients with FD).
Patients and controls included in the study were between 5 and 17 years old, eutrophic and with glomerular filtration rate (GFR) greater than 90ml/min/1.73m2. All with the approved medical consent.
Patients with diabetes, malnutrition with a Z score <2 SD, obesity, hyperparathyroidism, hyperthyroidism, primary alterations of metabolism, renal tubular acidosis and orthostatic proteinuria were excluded.
The children who presented a GFR reduction greater than 20% from inclusion in the study and those who did not comply with the indications for the collection of the sample were withdrawn from the study.
Variables analyzedWe identified as independent variables the absence of disease (control group), the presence of disease (FD), gender, and age of the patient at the time of admission. Dependent variables were the value of podocyturia, overt albuminuria (defined as albuminuria with values greater than 20μg/min), the time elapsed in months from the diagnosis of FD to the measurement of podocyturia. and the time elapsed from the diagnosis of FD to the appearance of overt albuminuria.
Laboratory studiesIn the Fabry group, blood was determined: serum creatinine, creatinine clearance (Scwhartz method), electrolytes, urea.
Twenty four hours urine was collected for measurement of albumin, creatinine, pH and urinary density.
PodocyturiaThe urine collected (24h) was used for laboratory analysis of both healthy and children with Fabry.
Samples were processed no more than 1h after emission, using a volume of 10ml to which 1ml of 10% formalin buffer was added in PB (pH 7.2–7.4). The samples were centrifuged at 1500rpm for 5min and the supernatant discarded. 10% formalin buffer was added to the sediment until it was covered and kept at room temperature until the study was completed.
An extended urine sediment was performed in xylanized slides of each sample.
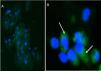
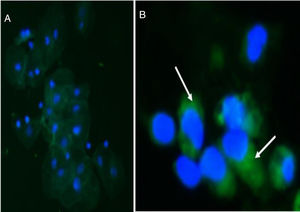
Indirect immunofluorescence was performed in a humid chamber. First, the samples were incubated with a blockade serum for 30min at room temperature. Then, without washing, it was incubated with the primary antibody antisinaptopodin (1:100, ABCAM, Cambridge, UK) used as a podocyte marker overnight at 4°C. Subsequently, there were washed 3 times with PBS, 5min each and incubated with secondary antibody Alexa 488 IgG (1:200, ABCAM, Cambridge, UK) for 2h at room temperature. Then the sample underwent, 3 washes with PBS, 5min each, and were stained with DAPI (ABCAM, Cambridge, UK). Samples were observed in a Nikon Eclipse E-200 epifluorescence microscope. The number of cells positive for synaptopodin was quantified in 10 fields 200× for each double-blind sample (Image Pro Plus 5.1, Media Cybernetics, Silver Spring, MD, USA).
Statistical analysisSample size calculation:
- a)
Percentage of patients with FD in whom it ws estimated to detect podocituria (p) 50% and another 50% without the alteration (q)
- b)
CI: 10%
- c)
Alpha error: 0.05, p: 95% (1.96)
- d)
Standard error: CI/1.96
Sample size: p×q 50×50=34
ES2 72
The quantitative determination of the variable podocyturia was made and the values were presented as continuous data, with its standard deviation in each group. Comparison of the mean quantitative differences of this continuous variable between the 2 groups was performed using with nonparametric test, the Mann–Whitney test.
The relationship between the values of albuminuria and podocyturia was evaluated using the Pearson correlation coefficient. The risk of developing albuminuria (dichotomous variable yes/no) was analyzed in patients with and without podocyturia (dichotomous variable yes/no), according to the relative risk.
Finally, for the association between potential risk factors and the appearance of pathological albuminuria, we used the Cox proportional hazards ratio. A p<0.05 value was established as a level of statistical significance.
The programs used as statistical supports were EPIDAT 3 and GraphPad inStat.
ResultsWe studied 31 patients, 11 with FD (5 female) and 20 controls (12 female), with an average age of 11.6 years.5–17
The mean time elapsed from the diagnosis of FD to the measurement of podocyturia (40 months) and to the appearance of overt albuminuria (34 months) was not significant (p=0.09).
The presence of synaptopodin was detected in podocytes from patients with FD and from the control group (Fig. 1) and the mean quantitative differences between both podocyturias were statistically significant (p=0.001).
The mean values of the urine measurements of are shown in Table 1.
Description of the measurements of urine parameters and other parameters in controls and patients with Fabry disease.
| Group A (control), n: 20 | Group B (Fabry disease), n: 11 | p | |
|---|---|---|---|
| Mean age, years (range) | 1.1 (5–17) | 1.7 (5–17) | 0.09 |
| Gender, M/F | 7/13 | 5/6 | 0.08 |
| Mean age in years at the diagnosis of FD (range) | 7.9 (5–11) | ||
| Mean GFR (ml/1.73m2/min)(SD) | 102 (8) | ||
| Proteinuria (mg/m2/h) (SD) | 1.5 (0.7) | 4.3 (0.5) | 0.05 |
| Albuminuria (ucg/min) (SD) | 10 (5.2) | 21 (12) | <0.01 |
| Pathological albuminuria (%) | 0 | 64% | <0.001 |
| Podocyturia/Cr in urine (mg/dl) (SD) | 0.4 (0.3) | 1.5 (0.8) | <0.01 |
SD: standard deviation; FD: Fabry disease; GFR, glomerular filtration rate.
In the Fabry group, not all patients had overt albuminuria. In fact, in 4 of them it was normal (Table 2).
Description of the variables collected in group B (Fabry disease).
| Gender | Age (years) | Podocyturia/Cr in urine (mg/dl) | Time elapsed from the diagnosis of FD to the measurement of podocyturia (months) | Albuminuria (ucg/min) | Time elapsed since diagnosis of FD to the observation of pathological albuminuria (months) | Mutation | Renal biopsy | Initiation and time on enzymatic treatment |
|---|---|---|---|---|---|---|---|---|
| Man | 17 | 0.5 | 59 | 25 | 59 | A292T | January 2012 | |
| Man | 16 | 0.8 | 85 | 33 | 85 | A292T | yes | (5 years of treatment) |
| Man | 10 | 2.3 | 29 | 39 | 29 | L415P | January 2012 (5 years of treatment) | |
| Woman | 15 | 1.1 | 46 | 27 | 29 | A292T | yes | March 2015 (2 years of treatment) |
| Woman | 12 | 2.2 | 35 | 26 | 23 | T194I | yes | February 2015 (2 years of treatment) |
| Woman | 12 | 2.6 | 18 | 17 | 18 | L415P | February 2017 (3 months of treatment) | |
| Man | 10 | 1.6 | 9 | 31 | 9 | – | October 2017 (7 months of treatment) | |
| Woman | 5 | 2.5 | 7 | 4 | – | – | October 2016 (1 year of treatment) | |
| Man | 12 | 1.6 | 32 | 6 | – | A292T | ||
| Man | 12 | 1.1 | 46 | 11 | – | A292T | ||
| Woman | 8 | 1.9 | 8 | 3 | – | A292T |
n: 11 patients; FD: Fabry disease.
The relative risk to develop pathological albuminuria according to the values of podocyturia was 1.1 in the control group (95% CI: 0.883–1.112) and 3.9 in the Fabry group (95% CI: 3.110–4.485). The correlation coefficient between podocyturia and albuminuria in the Fabry group was 0.8354 (95% CI: 0.4341–0.9601) (Fig. 2).
Finally, the 2 risk factors associated with the development of pathological albuminuria found in the Cox regression model were podocyturia (OR 14, 95% CI: 1.1–20) and age >10 years (OR 18; 95%: 1.1–39). We found no significant risk in the GFR (OR 0.5, 95% CI: 0.2–11), or gender (OR 1.3, 95% CI: 0.13–13).
During all controls, the average GFR remained within normal values (102ml/min/1.73m2).
The 7 patients with pathological albuminuria were started on nephroprotective treatment (enalapril 0.2mg/kg/day).
DiscussionThe present study evaluates 11 patients with FD and 20 controls. Our results indicate that in FD there is an early positive relationship between podocyturia and “overt” pathological albuminuria. To date, there are very few reports of FD in the pediatric population.8,9
In FD, the deficiency of the activity of the enzyme α-galactosidase A generates a progressive accumulation of lysosomas of glycosphingolipids and induces damage to the basement membrane with the consequent appearance of podocytes in urine.10
Indeed, it is known that podocytes coated by a glycocalyx sialoprotein are the main regulators of the physiological passage of proteins through the glomerular filter.
This filtration takes place through the diaphragm that links podocytes to each other and to the actin cytoskeleton.11–13 Many of the alterations in these structures are induced by the lysosomal accumulation of Gb3 and may result in pedicle effacement, detachment and development of pathological albuminuria.14,15 This sequence of events, at least initially, will justify, as proposed by Eng,16 the search for podocyturia as an adequate biomarker to evaluate early glomerular involvement of the disease. Therefore, its quantification would have a relevant prognostic value, representing an early, non-invasive indicator of kidney damage that may even precede the presence of proteinuria. Control of podocyturia should be evaluated, especially in those relatives in whom a confirmatory enzyme diagnosis has been made and no other renal involvement has yet been detected. Tondel et al. describes podocyte effacement and its subsequent detachment in most children with FD, so that both histological markers have been categorized as pathophysiological stages of early podocyte damage.17
Trimarchi et al. shows a high podocyturia with low proteinuria in patients without treatment. In our treated patients these findings seem to be reversed (with a more prolonged period of damage); this indicates a changing relationship between podocyturia and proteinuria as the disease progresses.18
In general terms, we can state that the magnitude of podocyturia (correcting per urine creatinine concentration) in the pediatric population, compared with adults, seems to confirm the theory of progressive podocyte reduction, related to age and the possible existing nephropathy. Consistent with this hypothesis was our observation in the younger patients with Fabry disease of an initial association between podocyturia and albuminuria, possibly related to the period of time with the disease, most obvious in a younger pediatric population. Hughes et al. reported, a relationship between age and the risk of developing kidney damage.19 In this same line, in FD patients the period of time elapsed between the detection of podocyturia and pathological albuminuria is similar, which support the assumption that a given time disease is required to see quantitative changes in pdocyturia and albuminuria.20
Patients with albuminuria were started on progressive doses of enalapril and most patients responded with a reduction of albuminuria. This data is not included in the manuscript.
The consensus among experts is the in patients with Fabry disease that enzyme replacement therapy should be started early; this may attenuate or prevent progressive nephropathy and other organic complications.21 However, according to our data the time elapsed until the evaluation of 2 key pathophysiological parameters (podocyturia and albuminuria) was too prolonged. This should be corrected if the intention is to apply effective therapeutic intervention. Nevertheless, there is no unanimity about the optimal time to start treatment and there is no long-term data documented as to whether the enzymatic prescription would prevent the nephropathy in children. A non-invasive method such as evaluation of podocyturia as an early marker of glomerular damage could help to decide the initiation of treatment.
The work has certain weaknesses, especially a reduced sample size and the impossibility of correlating the studies carried out with the pathological anatomy. However, the originality of the present work is the finding of an early positive association between 2 important variables such as podocyturia and albuminuria in pediatric patients. Our final aim, work in progress, is to establish the sequential relationship between both variables and determine the time interval of their successive appearances, so specific therapy is implemented early; this would be particularly beneficial in the Fabry population that has not yet developed the pathological albuminuria.
In conclusion, our study shows that the detection of podocyturia in pediatric patients with Fabry disease could be used as an early marker of renal damage, which precedes and is directly proportional to the appearance of pathological albuminuria.
Conflicts of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Liern M, Collazo A, Valencia M, Fainboin A, Isse L, Costales-Collaguazo C, et al. Podocituria en pacientes pediátricos con enfermedad de Fabry. Nefrologia. 2019;39:177–183.