Introducción: Se ha observado una relación entre el aumento de la transferencia de solutos (aumento del D/P de creatinina) y la disminución de la ultrafiltración, el aumento de la mortalidad y el riesgo de fracaso de la técnica en pacientes en diálisis peritoneal (DP). Las altas tasas de transporte de solutos se asocian con una mayor excreción peritoneal de proteínas (EPP) y esto se ha relacionado con un mayor riesgo de peritonitis. Nuestro objetivo fue evaluar la posible asociación entre la EPP, el número de episodios de peritonitis y el D/P de fósforo. Material y métodos: Se realizó un estudió longitudinal de cohorte prospectivo en pacientes en DP, a los que se les midió el D/P de fósforo, la EPP, el número de episodios de peritonitis, parámetros de adecuación, así como diferentes variables clínicas y bioquímicas. Resultados: Se incluyeron 60 pacientes en programa de DP ambulatoria. Se encontró una correlación significativa positiva (r = 0,369; p = 0,005) entre el D/P de fósforo y la EPP, al igual que entre la EPP y el número de episodios de peritonitis (r = 0,65; p = 0,044). Finalmente, se encontró que a mayor EPP y a mayor D/P de fósforo, menor nivel sérico de albumina (r = –0,50, p = 0,001 y r = –0,621, p = 0,000, respectivamente). Conclusiones: La EPP se asocia significativamente con el número de episodios de peritonitis y el D/P de fósforo.
Introduction: There is a relationship between increased transfer of solutes (increased D/P creatinine) and decreased ultrafiltration, increased mortality and risk of technique failure in peritoneal dialysis patients. High rates of solute transport are associated with increased peritoneal protein excretion (PPE) and this has been associated with an increased risk of peritonitis. Our objective was to evaluate the possible association between the PPE, the number of episodes of peritonitis and the D/P phosphate. Material and methods: A prospective longitudinal cohort study in PD patients. D/P phosphate, PPE, the number of episodes of peritonitis, as well as adequacy parameters and clinical and biochemical variables were measured. Results: We included 60 patients on ambulatory peritoneal dialysis. We found a significant positive correlation (r=.369, P=.005) between the D/P phosphate and PPE, as well as between the PPE and the number of episodes of peritonitis (r=.65, p=.044). Finally, we found that the higher PPE and D/P phosphate, the lower serum albumin was (r=–0.50, p=.001 and r=–0.621, p=.000, respectively). Conclusions: PPE is significantly associated with the number of episodes of peritonitis and the D/P phosphate.
INTRODUCTION
The technique survival in peritoneal dialysis (PD) continues to be lower than in haemodialysis (HD).1 Episodes of peritonitis and ultrafiltration failure (UF) are the main reasons for abandoning treatment.2
Peritoneal protein excretion (PPE) is universally regarded as an adverse effect of PD.3 The peritoneal transport of proteins occurs fundamentally through large intercellular pores and is limited rather by the size, than by load restriction.4 Plasma proteins are present in the peritoneal effluent of PD patients. This leads to a mean peritoneal protein loss of 5 to 10.5g per day in stable patients, but this may increase to 20g during an episode of peritonitis. Most of the proteins are albumin, however there are also others such as transferrin, immunoglobulin, complement proteins, ß2-microglobulin, and α2-macroglobulin.5,6
The PPE may operate as a marker of large pore dysfunction, suggesting systemic endothelial dysfunction, inflammation and, eventually, increased cardiovascular and peritonitis risk in patients with PD.3,7
The presence of a high rate of transport of small solutes may be a systemic or local manifestation of inflammation due to increased non-selective permeability of proteins during peritoneal inflammation with local generation of prostaglandins, mainly prostacyclin and prostaglandin E2.6,8
A relationship exists between the increase in solute transfer (D/P creatinine) and the decrease in UF over time on PD, which is also associated with increased peritoneal loss of proteins, hypoalbuminaemia and malnutrition. This process is exacerbated by episodes of peritonitis and has been associated with increased mortality and increased risk of technique failure in PD patients.9-13 Hypoalbuminaemia is considered a marker of comorbidity, rather than malnutrition.6
The D/P phosphate obtained by peritoneal equilibration test (PET) shows adequate correlation with the D/P creatinine. Some authors believe that creatinine clearance provides a good estimate of phosphate clearance, however, the first to categorise phosphate transfer in a population were Bernardo et al.,14 who observed that the distribution of the population in the different categories was different to the distribution presented for creatinine transport categorisation and as such they suggested that, despite the D/P phosphate having an adequate correlation with the D/P creatinine, the latter measure is not adequate enough to classify patients in accordance with the transport of phosphorus. Moreover, the D/P phosphate cannot be considered equivalent to the D/P creatinine, since there is no concordance between the two despite there being an adequate correlation, which was demonstrated in a study of our service by E. Lopez (personal communication currently under editorial revision), who found a low concordance between both parameters despite there being an adequate correlation.
The aim of the study was to assess the potential association between PPE, the number of episodes of peritonitis and the D/P phosphate in a Mexican population on continuous ambulatory PD.
MATERIAL AND METHOD
This is a prospective observational study which included prevalent PD clinic patients of the Instituto Nacional de Cardiología Ignacio Chávez, who were older than 16 years of age and who were on PD for at least a month before starting the study. Patients with active peritonitis event or who had completed antibiotic treatment within two months of the start of the study were excluded. We excluded patients who displayed ultrafiltration failure (UF below 400ml after the application of a 2000ml bag at 4.25% after 4 hours in the peritoneal cavity), patients with mechanical catheter problems (dialysate inlet time of 12 minutes and outlet time above 30 minutes), or those with a peritoneal fluid cellularity greater than or equal to 100 leukocytes per high power field at the time of the study.
All patients underwent modified PET15 using a 4.25% solution of glucose, having maintained a 1.5% solution in cavity for 8 to 12 hours previously. Baseline blood samples were taken for measurement of albumin, total protein, cholesterol, triglycerides and calcium, in addition to serum samples at 0, 2 and 4 hours in order to measure phospate and creatinine. Dialysate samples also were taken at 0, 2 and 4 hours and glucose, creatinine, phosphate and urea nitrogen were detected. At the end of the PET a dialysate cell count was performed to discard the active infectious process.
PPE was estimated from a complete collection of dialysate over 24 hours (expressed in g/24h). To quantify the total protein content in the dialysate, a modified colorimetric assay (pirogallol red) was used, which has proven an accurate method not affected by high concentrations of glucose in the dialysate.3,4
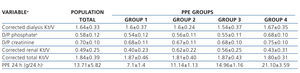
We calculated weekly the Kt/V of urea, phosphate clearance and creatinine adjusted to body surface area, both in their renal, peritoneal and total form, in addition to the weight-adjusted protein equivalent of nitrogen (nPNA).
The type of peritoneal transport of solutes was classified according to creatinine16 and phosphate transport.
The Kt/V, nPNA and phosphate clearance were obtained using the following formulae:
1) Kt/V weekly urea = 7 x (CE x EV/CB x t)/V, where CE is the urea concentration in the effluent, EV is effluent volume, CB is serum urea concentration and V is distribution volume in litres; Watson formula by gender:
Men = 2.447 - (0.09156 x age) + (0.1074 x height cm) + (0.3362 x weight kg)
Women = -2.097 + (0.1069 x height cm) + (0.2466 x weight kg)
2) Phosphate clearance = (PO4 concentration [dialysate or urine] [in mg/dl]/ PO4 serum in mg/dl) x dialysate drain volume x 7 (corrected to 1.73 m2 body surface area (BS) and expressed in litres/week).
3) Creatinine clearance = (creatinine concentration [dialysate or urine] [in mg/dl]/serum creatinine in mg/dl) x dialysate drain volume x 7 (corrected to 1.73 m2 BS and expressed in litres/week).
4) nPNA = Randerson formula
34.6 + (5.86 x [(BUN DL x vol DL 24 h) + (urinary BUN x urine volume 24 h)]) where BUN is blood urea nitrogen and DL is dialysate.
Urea distribution volume (Watson formula)/0.58
Patients were followed up for an 8 month period with the number of peritonitis episodes during this period being documented. As an indicator of comorbidity the Charlson index17 was used.
STATISTICAL ANALYSIS
Results are expressed as mean ± standard deviation or as proportions, as appropriate. Mean comparison was performed with independent t test or single factor ANOVA samples in the case of more than two groups. In accordance with the distribution of each variable, in some cases non-parametric alternatives to the above tests were employed (U Mann-Whitney or Kruskal-Wallis ANOVA). Comparison of proportions was performed by χ2 test. The search for association between two variables was performed using Pearson’s correlation coefficient. For purposes of segmentation and classification of some variables, the optimal intervals tool was used or a CHAID (Chi Squared Automatic Interaction Detector) method classification tree was created. A value of P<.05 was considered significant. The statistical package SPSS version 15 for Windows was used.
RESULTS
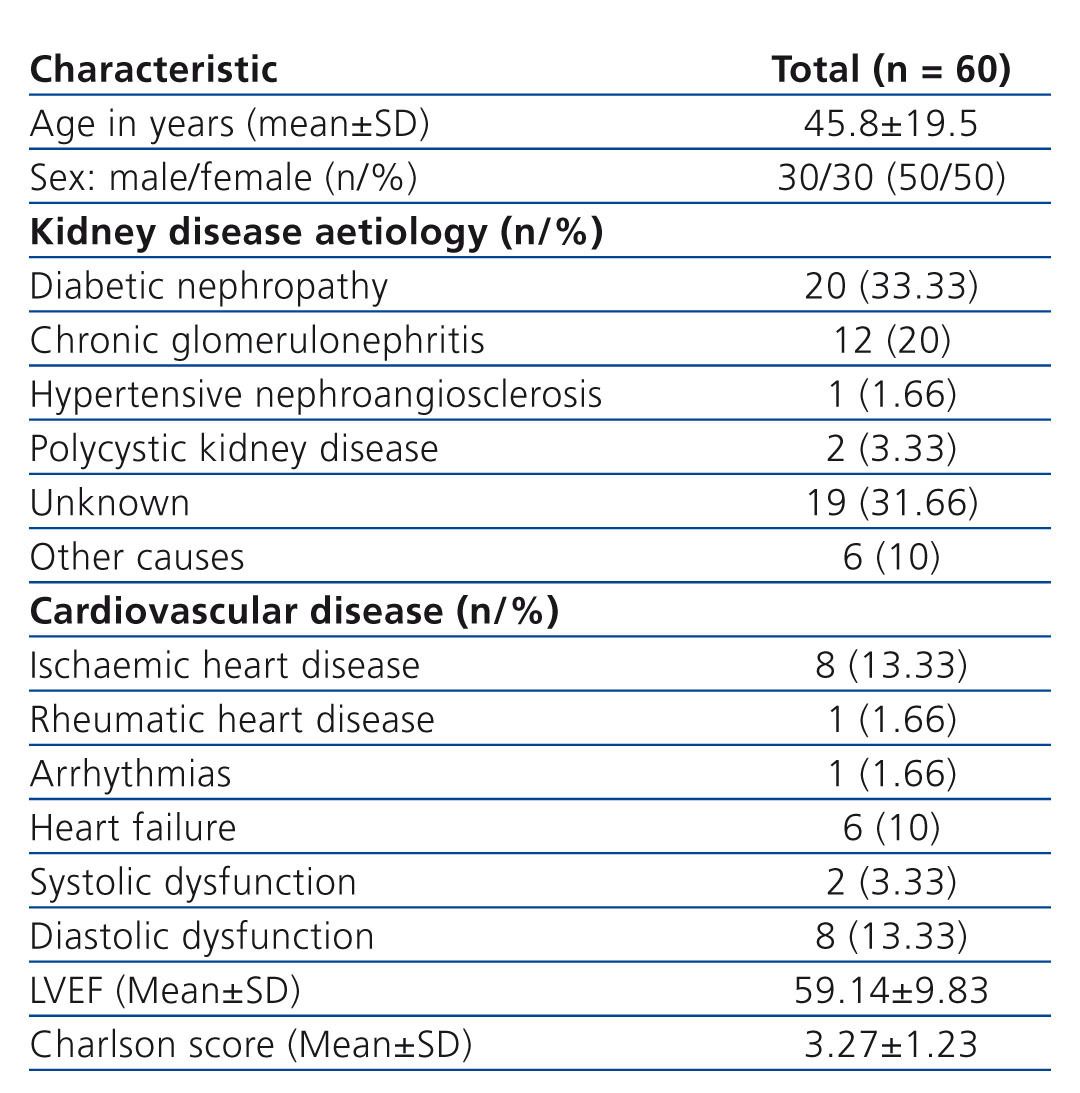
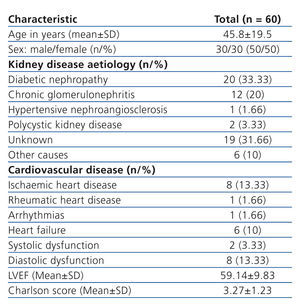
The study included 60 patients from the PD clinic. Only 2 patients were excluded from the final analysis because they were lost to follow-up. Of the patients who started the study, 30 were female (50%), with a mean age of 45.8±19.54 years (Table 1). Biochemical baseline values for the total group were: serum creatinine 11.26±4.05mg/dl, BUN 54.28±14.57mg/dl, alkaline phosphatase 140.35±90.46U/l, calcium x phosphate 50.13±16.05, and C-reactive protein (CRP) 7.3±9.8mg/dl.
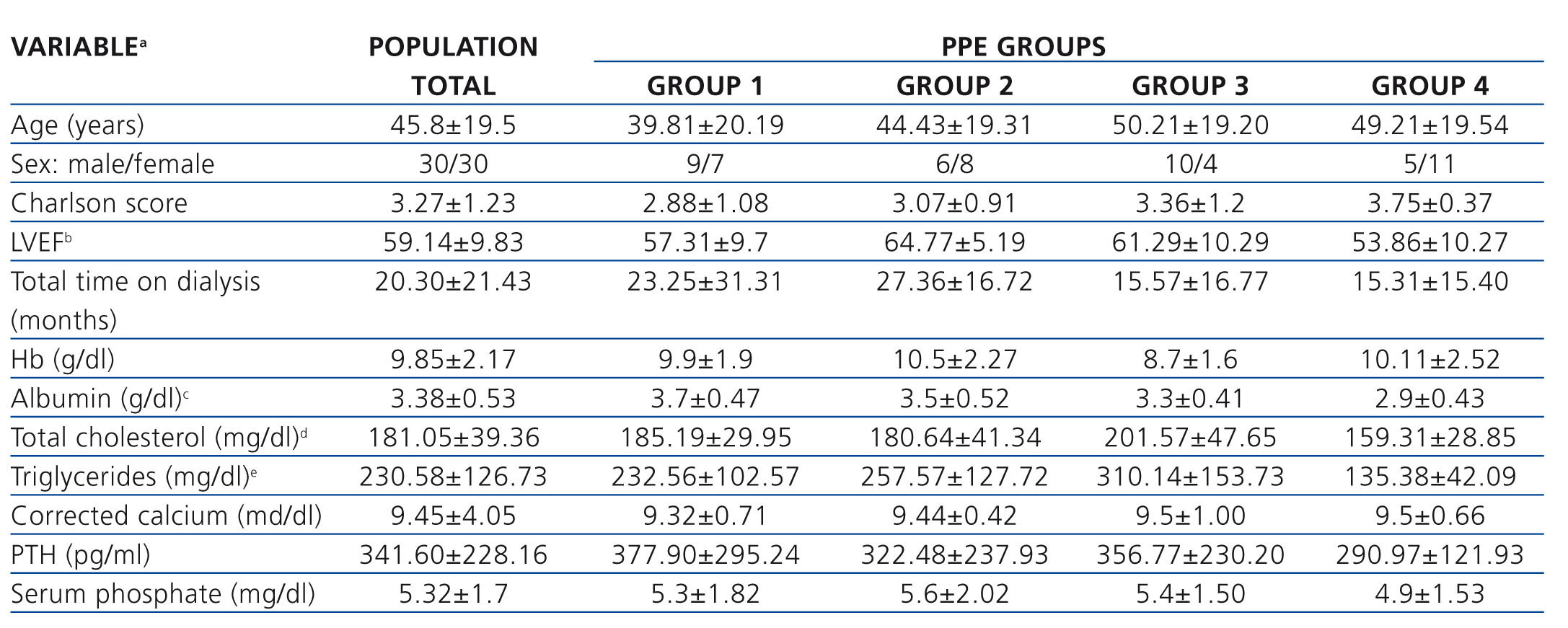
The total study population was divided into four groups, in accordance with the total PPE in 24 hours, using the method of optimal intervals: group 1: ≤ 9.11 g/24h, group 2: 9.12 to 12.66g/24h, group 3: 12.67 to 17.39g/24h group 4: ≥ 17.40 g/24h. Also as part of the analysis, and using the same optimal interval strategy, patients were divided according to the serum albumin value (albumin ≤3.4mg/dL albumin >3.4mg/dl).
When analysing PPE groups, we found no significant difference in the distribution of age, sex and Charlson comorbidity score.
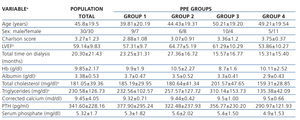
Regarding the value of the left ventricular ejection fraction, we found a significant difference between group 2 and group 4, with group 2 being higher. In the case of serum albumin, the difference was found between group 1 and group 4, with group 4 being lower. Lastly, for cholesterol and serum triglycerides, there was a significant difference found between group 3 and group 4, with group 3 having higher values. The rest of the clinical and laboratory characteristics are described in Tables 2 and 3 without other significant differences having been identified between groups.
Cases of peritonitis
During follow-up, 18 patients had at least one episode of peritonitis, 10 patients had a single episode, 5 patients two episodes and 3 patients three episodes of peritonitis, corresponding to 16.7%, 8.3% and 5% respectively. Thirty-nine patients had no episode of peritonitis (66.7%). When comparing the number of episodes of peritonitis by sex, we found a significantly higher number in males (12 vs. 6, P=.000).
Peritoneal protein excretion and episodes of peritonitis
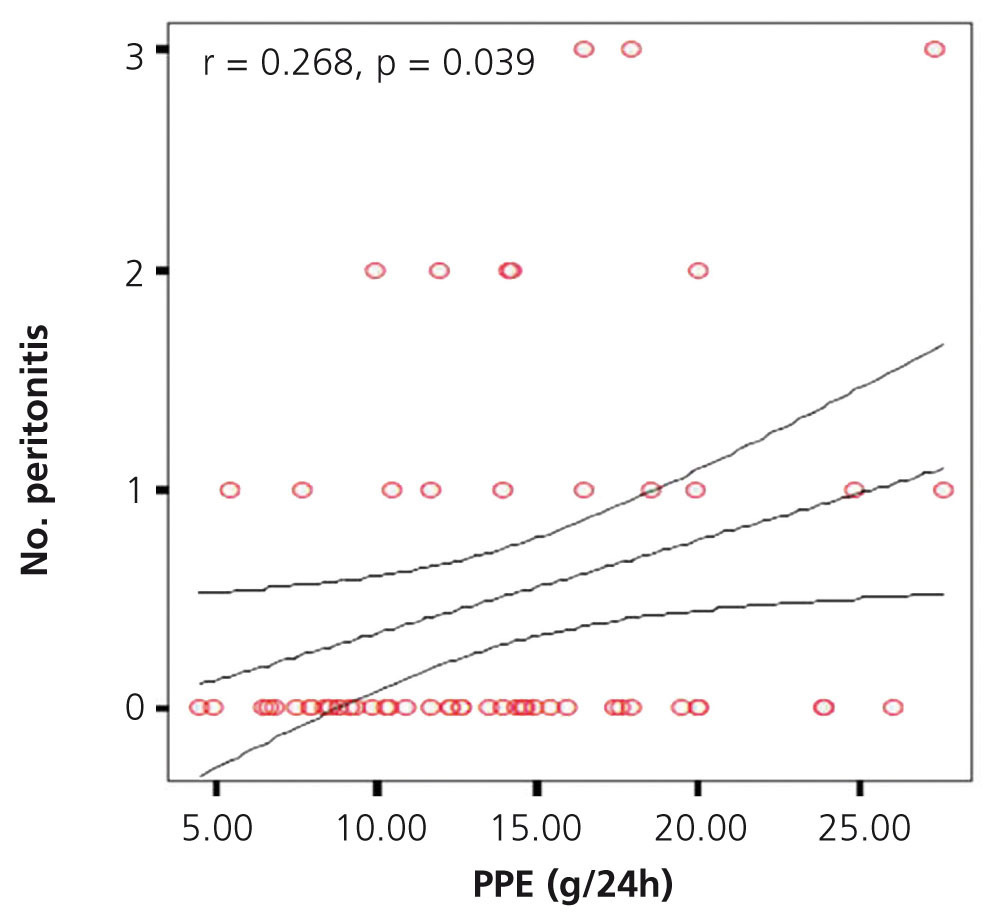
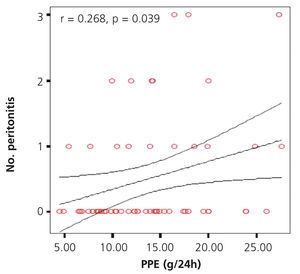
We investigated the possible association between PPE in 24 hours and the number of events of peritonitis, finding a significant positive correlation (r=0.65, p=.044), as shown in the graph (Figure 1). No significant difference was found when comparing the different PPE groups with the number of episodes of peritonitis. However, there was an upward trend in the number of peritonitis episodes in the groups with higher PPE, with a greater difference between group 1 and group 4 (2 in group 1 vs. 7 in group 4) being found.
Category of creatinine and phosphate transport in relation to the cases of peritonitis
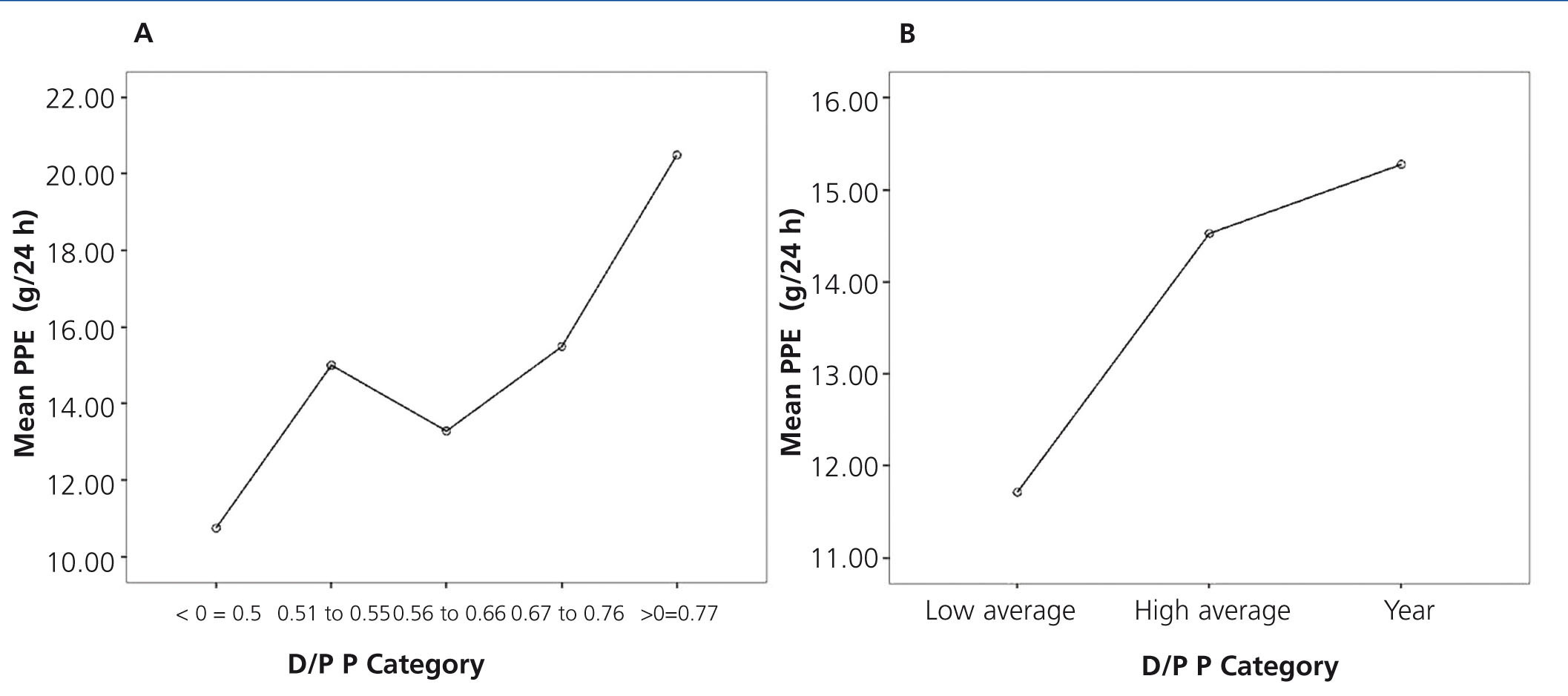
As another part of the analysis, the total study population was divided in accordance with the categories defined by the parameters used in solute transport. In the case of D/P creatinine, 4 groups were considered in accordance with that proposed by Twardowski. With respect to the D/P phosphate, we used 5 groups obtained using a classification tree, as follows: group 1 (≤0.50), group 2 (0.51 to 0.55) group 3 (0.56 to 0.66), group 4 (0.67-0.76) and group 5 (>0.76).
The number of episodes of peritonitis was not significantly different between the groups according to the categories of transport for both creatinine and phosphate.
The association between the D/P phosphate and the number of episodes of peritonitis, although not significant, did show a trend (r=0.23, p=.094). On evaluating the association between the D/P creatinine and the number of episodes of peritonitis, no significant correlation was found (r=0.21, p=not significant).
D/P creatinine and D/P phosphate in relation to peritoneal protein excretion
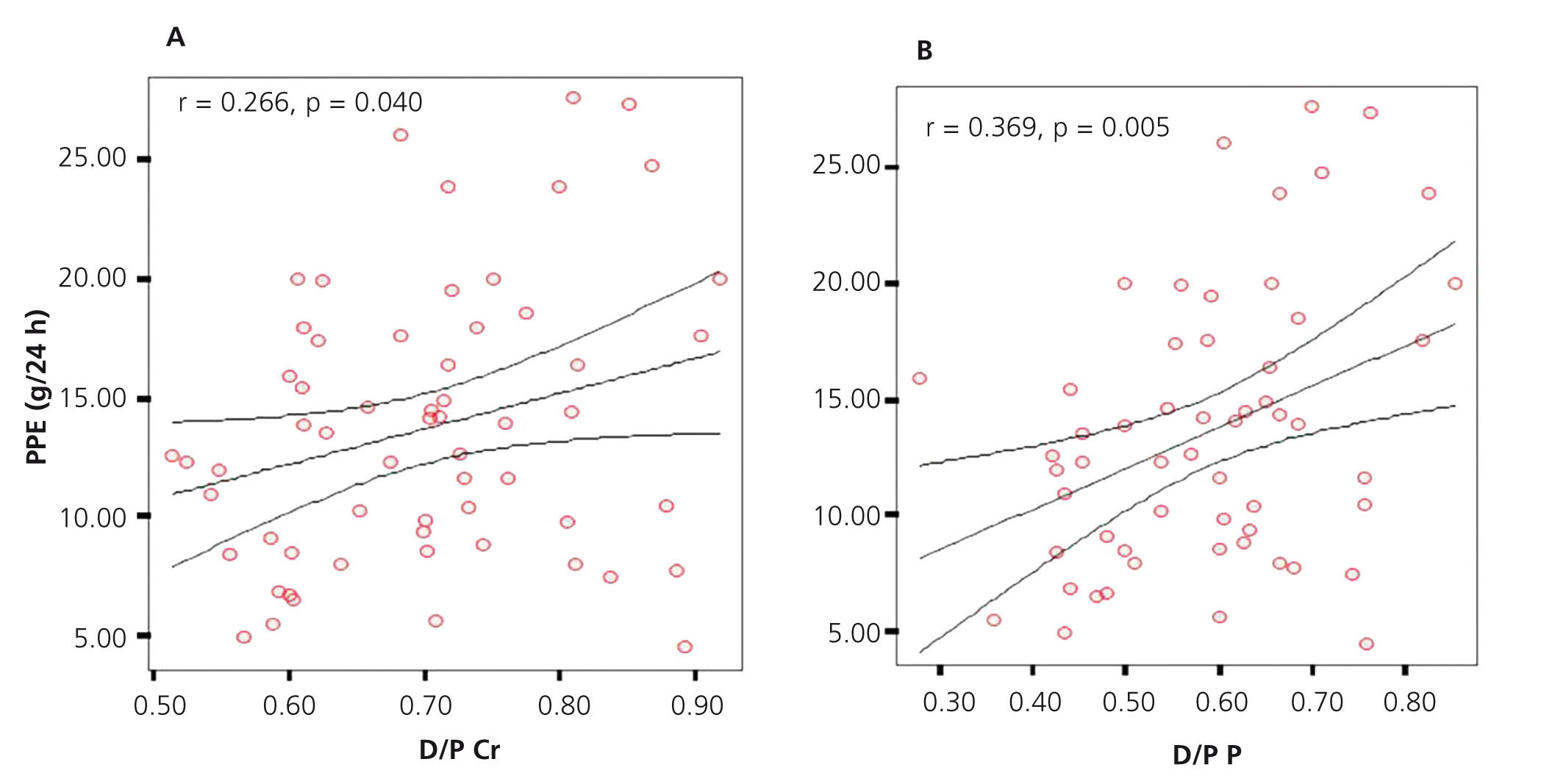
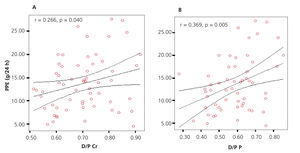
Applying Pearson’s association test, we found a significant positive correlation (r=0.266, P=.040) between D/P creatinine and PPE. Applying the same association test, but this time between D/P phosphate and PPE, we found a greater and significant strength of association (r=0.369, P=.005) (Figure 2A and B).
Peritoneal protein excretion groups and the creatinine and phosphate transport category
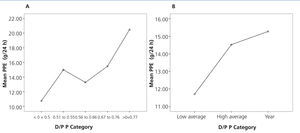
On comparison of the PPE between groups according to creatinine transport, no significant differences were found. However, there was an upward trend in PPE values in quicker creatinine transport groups. Moreover, significant differences were found (P=.042) when comparing the PPE between phosphate transport categories, with higher PPE in high transport groups (Figure 3A and B).
Comorbidities and peritoneal protein excretion groups
With regard to comorbidities, the proportion of cases with ischaemic heart disease was not significantly different between PPE groups, but there was a trend in a higher percentage of cases of ischaemic heart disease in the groups with higher PPE. There were no significant differences identified when comparing the percentages of patients with systolic or diastolic dysfunction or cerebrovascular disease (CVD) by PPE group.
Diabetes and survival
21 cases had diabetes, corresponding to 35% of the total sample of patients studied. On comparing survival between diabetic and non-diabetic patients, no significant difference was found.
The proportion of diabetic patients was significantly different between PPE groups, being higher in groups with high PPE.
Total peritoneal dialysis time and peritoneal protein excretion
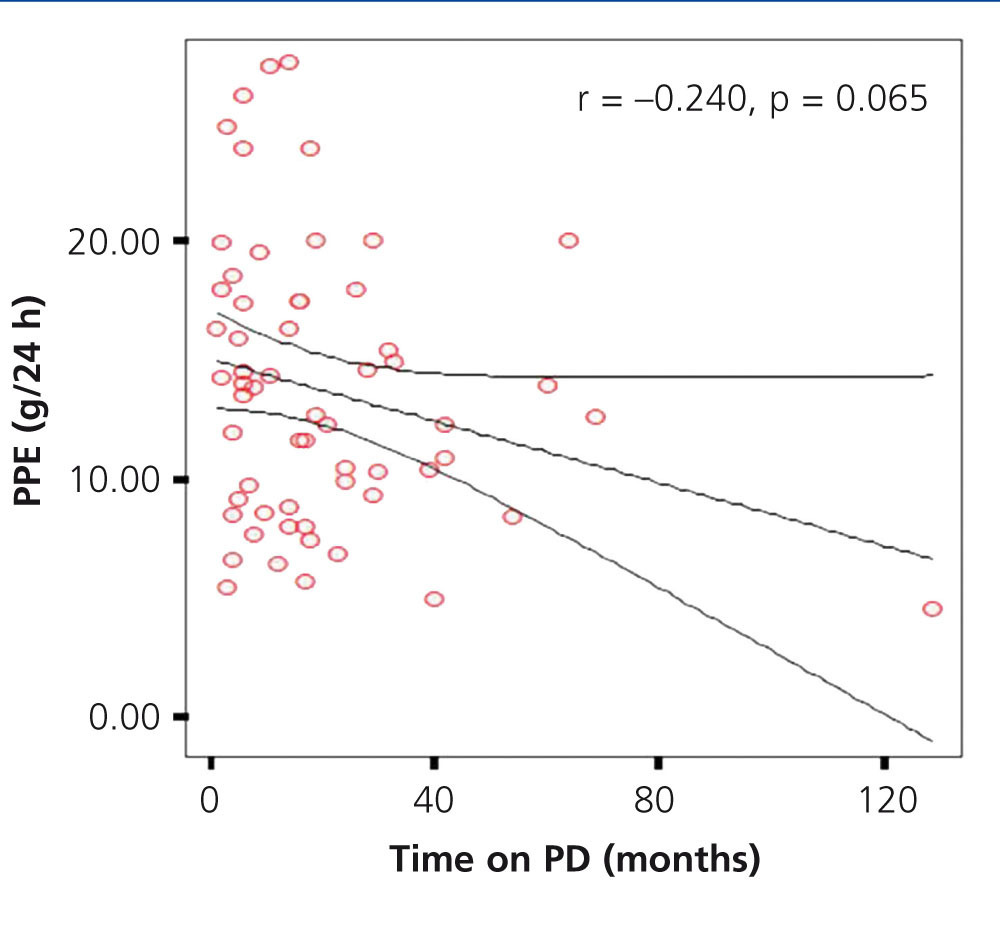
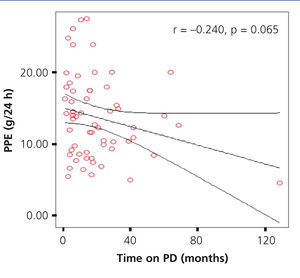
In our study, a mean time on PD for the total population of 20.30±21.43 months (range 1-129) was observed. In assessing whether there was an association between the time of dialysis and the PPE, we found an insignificant negative correlation (r=-0.240, P=.65), however, we observed a lower PPE trend in patients who spent more time on dialysis (Figure 4). On comparing the time on dialysis of PPE groups, there were no significant differences.
Number of peritonitis and total time of dialysis
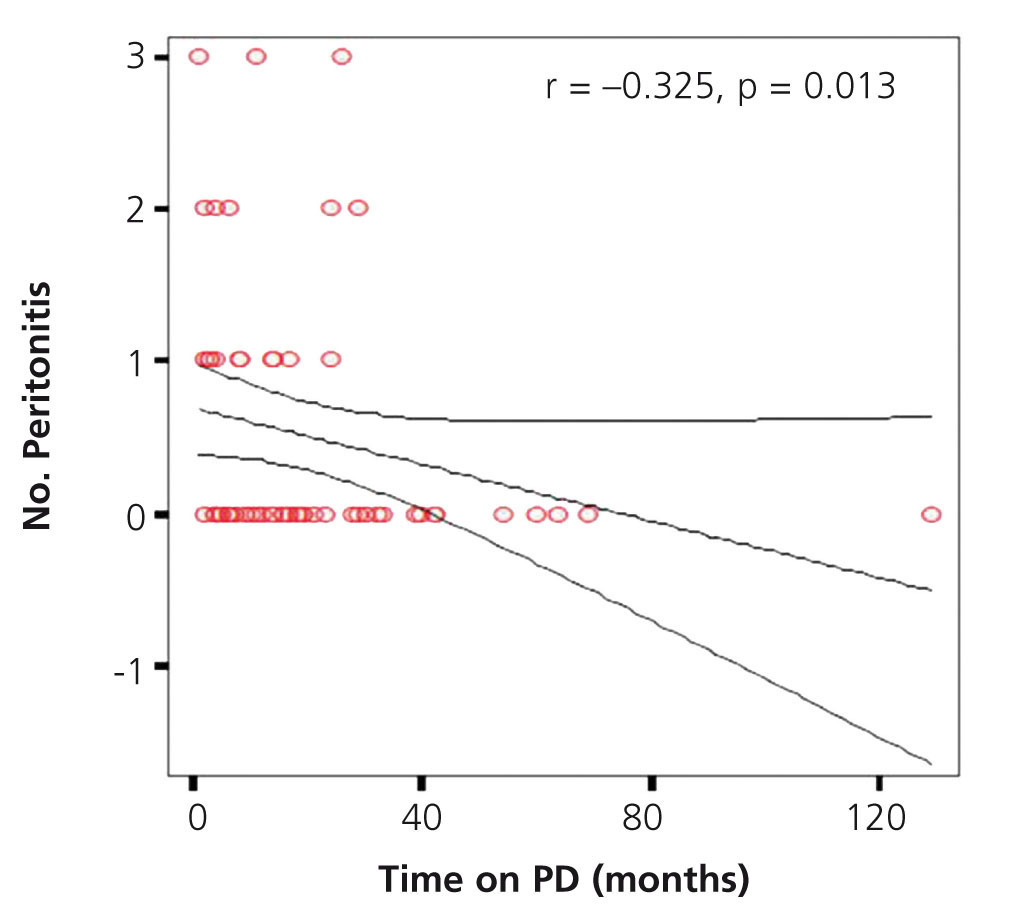
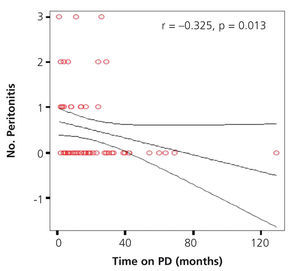
We found a significant negative correlation between the total time spent on dialysis and the number of episodes of peritonitis (r=-0.325, P=.013) (Figure 5).
Association of albumin with different variables
The mean value of serum albumin in the total sample of patients was 3.38±0.53g/dl (range 2.1 to 4.5). For comparative purposes and in accordance with the distribution of serum albumin values, we created two groups: group 1, serum albumin ≤ 3.4g/dl, and group 2, serum albumin> 3.4g/dl. In evaluating the PPE between these groups, it was found that the group with the lowest value of serum albumin had a significantly higher PPE than the group with the highest serum albumin value (P=.001). We also found a significant difference between serum albumin groups when comparing D/P creatinine and D/P phosphate, both being lower in the group with higher serum albumin (P=.000 for both variables). We found no significant difference between groups of albumin with respect to time spent on dialysis.
On comparing between albumin groups the proportion of patients in each creatinine transport category, we found that the proportion of patients with high transport was significantly higher in the group with the lowest albumin (p=.006). For the case of different phosphate transport categories, the proportion of patients in the two highest categories of transport was also significantly higher in the group with the highest serum albumin (p=.002).
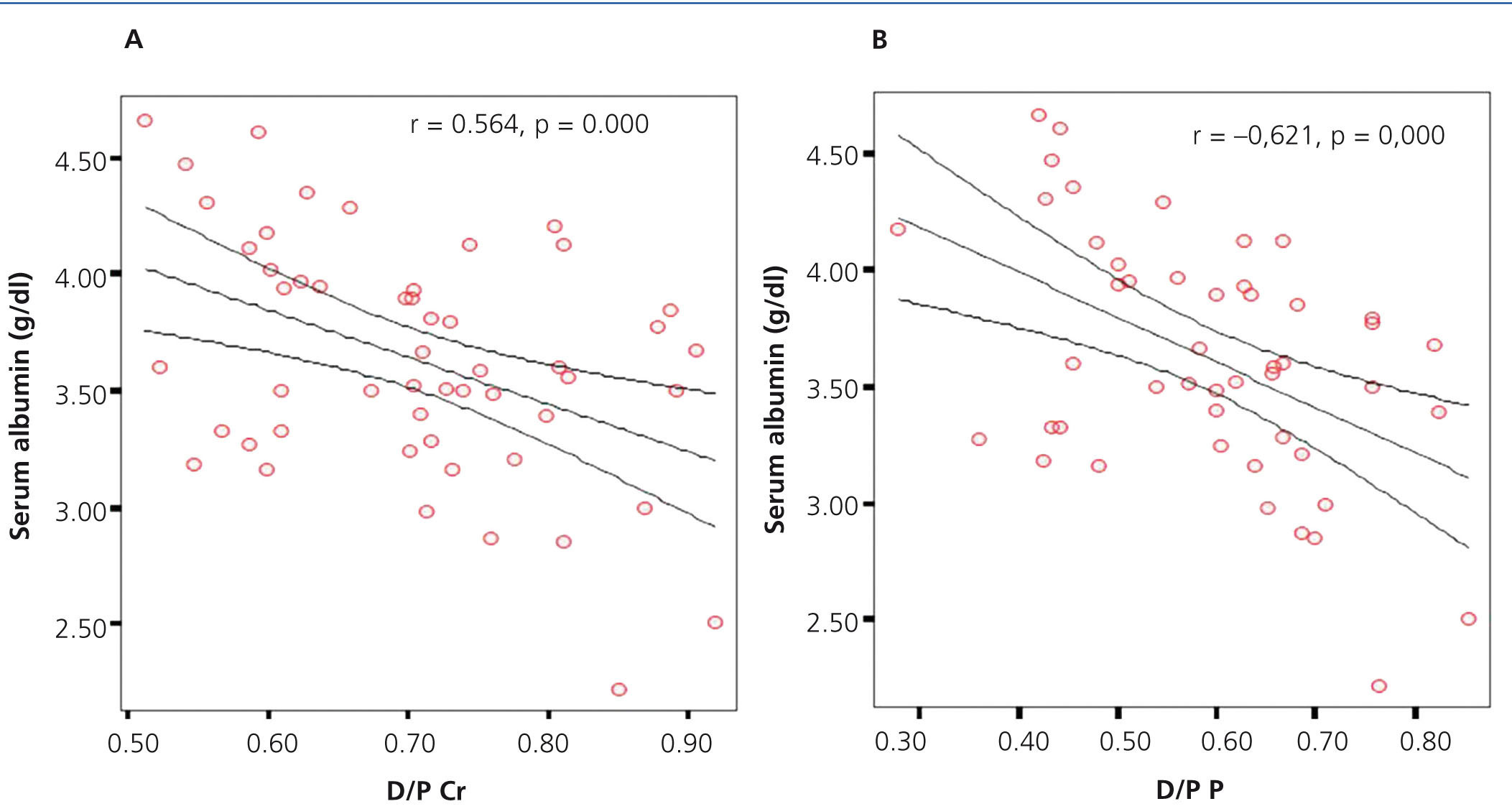
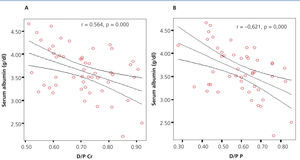
In assessing the possible association between the value of serum albumin and D/P creatinine and D/P phosphate, we found, in both cases, a significant negative correlation (r=-0.564, p=.000 and r=-0.621, p=.000 for D/P creatinine and D/P phosphate, respectively), observing for a higher amount of serum albumin, lower D/P creatinine and D/P phosphate (Figure 6 A and B).
Final outcome
Of the 60 patients included in this study, we found that at the end of the follow-up, 47 patients (80%) remained on PD, 5 patients (8.33%) died, 3 patients (5%) transferred to HD modality due to cavity loss secondary to peritonitis, 3 patients (3.33%) underwent transplantation and 2 patients (3.33%) were lost to follow-up.
DISCUSSION
The major problem of PD patients today remains the loss of useful peritoneal cavity. Peritonitis episodes and UF failure are the major causes of treatment discontinuation. It has been shown that a single episode of peritonitis can result in morphological and functional changes of the peritoneal membrane. Peritonitis is associated with a decrease in net UF, increased solute clearance, increased glucose uptake and an increase in the peritoneal loss of proteins. The association between PPE and the number of episodes of peritonitis is associated with a greater loss of the peritoneal cavity and, therefore, is one of the main causes of dialysis modality change.2
In our population, we found an incidence of peritonitis episodes 18% below that reported in literature, which has estimated that 45% of patients have peritonitis at least once during the first six months of treatment with continuous ambulatory PD (CAPD) and the rate increases to 60-70% during the first year. A recurrence of 44% was observed, which is higher than that reported in other series, which is between 20 and 30% and it is one of the most common reasons for discontinuing CAPD.
In three of our patients in the study, it was necessary to change the modality of CAPD to HD. In assessing the cause of this change, we found that in all three cases it was due to recurrence of peritonitis, which caused dysfunction of the peritoneal cavity.
When comparing the incidence of peritonitis by sex, we found a higher frequency both in episodes and in recurrence of peritonitis in males, (66.66% and 62.5%, respectively). These data are similar to those of other studies, where a frequency of up to 55% is reported.
PPE values found in this study were similar to those reported in other publications,5,18,19 and our results confirm the recognised association between PPE and the number of peritonitis episodes in PD patients. We also observed that in higher PPE, the frequency of peritonitis is higher.
In evaluating the time spent on PD with regard to PPE and episodes of peritonitis, we found that patients who spent more time on dialysis had fewer episodes of peritonitis and less PPE, although the latter was not statistically significant. This suggests that structural and functional changes occur within the peritoneal membrane.2
The high transport status of small solutes from the peritoneal membrane, as defined by a standard PET, has been associated with an increased mortality and technique failure in PD patients. This type of transport is strongly associated with genetics, inflammation, a large vascular peritoneal surface and, in particular, with comorbidity conditions. Peritoneal membrane damage may also be associated with the time spent on dialysis, as well as with the use of bioincompatible dialysis solutions and the number of episodes of peritonitis.20
Consideration has been made of the hypothesis that patients with high solute transport rates may have increased protein loss in the dialysate, which would lead to the development of hypoalbuminemia and malnutrition. There is also evidence to suggest that patients with this type of transport have a less effective UF, resulting in volume expansion and dilutional hypoalbuminemia.13 This was also observed in our study, since patients who belonged to the least significant albumin group were associated with a higher D/P creatinine and phosphase and with higher PPE.
In our population we found a greater transfer of solutes in patients with higher excretion of protein, observing a better association of the PPE with D/P phosphate, compared with D/P creatinine. We found that the D/P phosphate was lower than D/P creatinine in the four PPE groups. A possible explanation for this is the way that phosphate is distributed in the body, since about 5 to 10% of plasma phosphate is fixed to proteins, unlike creatinine, which is not linked to proteíns.21 In the case of PPE group 4, we found that the value of D/P phosphate is closer to D/P creatinine. In assessing this phenomenon, we found that the group with higher PPE was associated with lower levels of serum albumin, which could explain the smaller difference between the values of D/P creatinine and D/P phosphate, since reducing the serum proteins also decreases the amount of phosphate that is protein-bound. An association was also observed between the D/P phosphate and the number of episodes of peritonitis and, although this finding was not statistically significant, there was a tendency for more episodes of peritonitis at higher D/P phosphate levels.
We have identified some variables associated with prognosis of peritoneal function, among which are PPE, the type of peritoneal solute transport, the number of episodes of peritonitis, time on dialysis, and while the D/P creatinine has also been considered as a prognostic marker, we observed that the D/P phosphate had a better correlation than D/P creatinine with PPE and the number of episodes of peritonitis, and as such, this parameter could be considered as a new prognostic evaluation tool as well as being used for the purpose of dialysis adequacy. This should be evaluated in a study with a larger sample and long-term follow-up.
In regard to the subpopulation of patients with diabetes mellitus, we found that these patients had higher PPE. This increased peritoneal loss of proteins in diabetic patients seems to be related to high peritoneal transport of solutes. Previous studies have suggested that this increased permeability of the peritoneal membrane to proteins could be a result of diabetic microvascular lesions in a pattern similar to that observed in glomerular membrane damage.22
A targeted study was conducted with regards to whether the episodes of peritonitis that patients displayed prior to carrying out the study had some connection with episodes during the study, as well as their relationship with hypoalbuminaemia and D/P phosphate, finding that of the 60 patients studied only 12 had had previous episodes of peritonitis and these episodes had occurred more than two months before starting the study. Of these 12 patients, only 3 had one or more episodes of peritonitis during the study follow-up and two of these patients required dialysis modality change to HD due to loss of the cavity. Only 4 patients of the 12 cases with previous episodes of peritonitis had serum albumin below 3.4g/dl and 6 had a high D/P phosphate. We did not identify a significant association between the number of previous episodes of peritonitis and the serum albumin level or D/P phosphate.
There are two main limitations to our study. The first is the limited sample size and the second is the short follow-up time. Another limitation to our study is the type of population studied, since it included only 35% of patients with diabetes, compared with other studies, which have included up to 50%.
We conclude that the PPE is significantly associated with the number of episodes of peritonitis and with the value of serum albumin. We observed a significant association between the PPE and D/P phosphate and between the latter and serum albumin. D/P phosphate had a better association with the number of peritonitis episodes compared to D/P creatinine.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Sponsorship sources
None. Carried out with own funds.
Table 1. Study population: baseline clinical characteristics
Table 2. Baseline clinical and biochemical characteristics of the total group and for peritoneal protein excretion
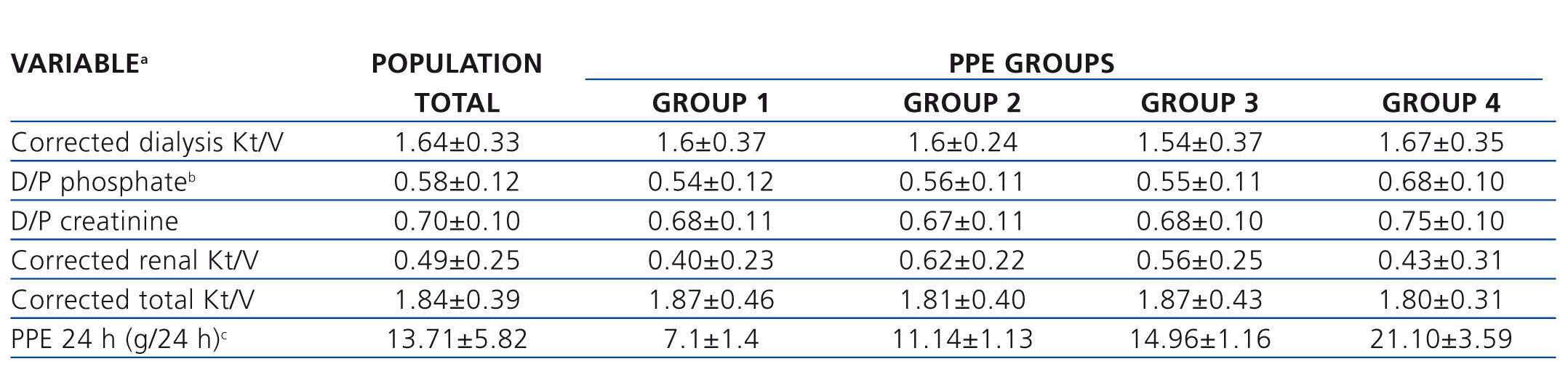
Table 3. Characteristics of baseline adequacy of the total group and for peritoneal protein excretion
Figure 1. Graph showing correlation between peritoneal protein excretion and the number of peritonitis episodes
Figure 2. Graph showing correlation between D/P and peritoneal protein excretion
Figure 3. Mean graph for peritoneal protein excretion according to the transport category
Figure 4. Correlation between time on dialysis and peritoneal protein excretion
Figure 5. Correlation between total time on dialysis and the number of episodes of peritonitis
Figure 6. Correlation between serum albumin and D/P