Antecedentes y objetivos: SureClick® es una pluma precargada para la administración de darbepoetina alfa (DA), que está lista para usar. Se exploró la satisfacción del paciente con SureClick® en comparación con las jeringas precargadas. Métodos: Estudio observacional multicéntrico, prospectivo, de 6 meses, en el que se incluyeron pacientes no dializados con enfermedad renal crónica (ERC) tratados con DA en jeringas precargadas que cambiaron a SureClick® al inicio del estudio. Las principales variables fueron: cambios en el Cuestionario de Satisfacción con el Tratamiento de la Anemia (ATSQ-S), Escala de Competencia Percibida en el Manejo de la Anemia (PCAS) y el porcentaje de autoadministración. Resultados: Se incluyeron 132 pacientes con una media (± desviación estándar [DE]) de 71,3 (14,6) años y un 57,6 % de mujeres. Las puntuaciones basales y finales medias (DE) en la ATSQ-S fueron, respectivamente, 25,5 (7,9) y 31,6 (4,9) (en una escala de 0 a 36-máxima satisfacción, cambio medio: 6,2, intervalo de confianza [IC] 95%: 4,6-7,8, p < 0,0001). La puntuación de la PCAS también aumentó significativamente (4,3 [2,0] vs. 5,6 [1,6], en una escala de 1 a 7-máxima competencia, p < 0,0001). Al inicio del estudio, el 47,7 % de los pacientes se autoadministraba DA con jeringas precargadas, frente al 74,2 % con SureClick® (p < 0,001). No hubo cambios significativos en el nivel medio de hemoglobina (11,4 [0,5] vs. 11,6 [1,3] g/dl, p = 0,193). Dos pacientes (1,5 %) presentaron reacciones adversas a SureClick® (dolor en el lugar de aplicación). Conclusiones: Los resultados sugieren que el cambio de jeringas precargadas a SureClick® podría aumentar la satisfacción del paciente y la percepción de la competencia en el manejo de la anemia en pacientes con ERC no dializados, y podría aumentar la tasa de autoadministración, lo que reduciría el uso de recursos sanitarios.
Background and aims: SureClick® is a prefilled pen for administration of darbepoetin alfa (DA) that is ready-to-use. We explored patient satisfaction with SureClick® compared with prefilled syringes (PFS). Methods: Multicenter, prospective, 6-months, observational study in non-dialyzed patients with chronic kidney disease (CKD) treated with DA in PFS who switched to SureClick® at baseline. Main outcomes were: change in Anemia Treatment Satisfaction Questionnaire (ATSQ-S), Perceived Competence for Anemia Scale (PCAS) and self-administration rate. Results: We enrolled 132 patients with a mean(SD) age of 71.3 (14.6) years, 57.6% women. Mean(SD) ATSQ-S scores at baseline and final records were 25.5 (7.9) and 31.6 (4.9) (on a scale from 0 to 36 –maximum satisfaction-, mean change: 6.2, 95%CI: 4.6-7.8, p<0.0001). The PCAS also increased significantly (4.3 (2.0) vs 5.6 (1.6), on a scale from 1 to 7 –maximum competence, p<0.0001). At baseline 47.7% of patients self-administered DA with PFS, vs 74.2% with SureClick® (p<0.001). No significant changes in hemoglobin were observed (11.4 (0.5) vs 11.6 (1.3) g/dl, p=0.193). Two patients (1.5%) had adverse reactions to SureClick® (pain on application). Conclusions: Our results suggest that the change from PFS to SureClick® could increase patient satisfaction and perceived competence in anemia management in non-dialyzed CKD patients, and could increase the self-administration rate, thereby reducing use of health resources.
INTRODUCTION
Educating patients for self-administration, i.e. increasing patient’s “competence”, can have an impact on disease progression and quality of life.1 Self-managed diabetic patients obtain better glycemic control than those whose health care providers are not autonomy-supportive.2,3 Patient education programs in renal anemia associated to chronic kidney disease (CKD) may lead to a concomitant reduction in nursing time and associated costs.4 The development of easy-to-use subcutaneous (s.c.) delivery devices increase the patient availability to assume the self-administration. Currently, there are four s.c. administration options for erythropoiesis-stimulating agents (ESAs): multi-dose vials plus traditional syringes, prefilled syringes, multi-use pens plus cartridges and single-use prefilled pens.
SureClick® (for darbepoetin alfa (DA) administration) is the only single-use, ready-to-use, prefilled pen approved for ESA administration in the European Union (EU). The other marketed autoinjector, Reco-Pen® (for epoetin beta administration),5 is a multi-use system which requires some previous manipulation. In addition to its convenience of use, the SureClick® device has a hidden needle and appears to be safe.6-8 Previous studies performed in anemia with multi-use injectors (Reco-Pen®) demonstrated an improvement in patient´s satisfaction.9,10 The present study aimed to explore patients’ satisfaction, perceived competence and degree of self-administration with SureClick® in patients with CKD not on dialysis on stable treatment with DA.
METHODS
Study population
The cohort was sampled from patients receiving outpatient predialysis care. To minimize sample bias, all consecutive patients attending the clinics who fulfilled eligibility criteria were invited to participate. Due to the fact that the only marketed anemia treatment that can be administered with single-use prefilled pens is DA, the source population was limited to patients routinely treated with this drug. The inclusion criteria were: patients aged ≥18 years with CKD not undergoing dialysis, treated with DA by s.c. route using prefilled syringes for at least 5 months; patients who had received previous self-administration training with prefilled syringes; stable patients (DA dose variation <10% and hemoglobin (Hb) levels between 9.5 to 12.5g/dL, with a DA administration frequency every-2-weeks (Q2W) during the previous 2.5 months); patients to whom the SureClick® was clinically indicated; and patients who gave their written informed consent. Patients with previous kidney transplant were excluded.
Study design
We conducted a prospective, multicenter, observational, cohort study in 16 Spanish nephrology units in 2009. Centers were selected based on their use of both SureClick® and prefilled syringes in the clinical practice. During the selection procedures, we verified that all of them trained non-dialyzed CKD patients to self-administer anemia medication in their routine care. Participating units belonged to large or medium-sized hospitals geographically distributed throughout Spain, which suggests that our sample was reasonably representative of the entire population of Spanish CKD patients. At baseline (Record 0), all patients switched from prefilled syringes to SureClick®, and the hospital personnel performed face-to-face self-administration training in using SureClick®. Data were collected at baseline and at two subsequent records (Records 1 and 2) at approximately 3 and 6 months.
All patients received s.c. DA (Aranesp®, Amgen Inc., Thousand Oaks, CA, USA), with the SureClick® device according to the Summary of Product Characteristics.
Outcomes
The primary outcome was the mean change from baseline to the last assessment in Anemia Treatment Satisfaction Questionnaire, status version (ATSQ-S). Secondary outcomes included: mean change in Perceived Competence for Anemia Scale (PCAS), mean ATSQ-C (change version) at Record 2, change in self administration rate, change in Hb levels, compliance (defined as the percentage of prescribed injections that were actually administered) and incidence of adverse reactions to DA or device.
Psychometric measures
Satisfaction was measured with the ATSQ (status and change versions, Appendix 1 and Appendix 2), adapted from the Diabetes Treatment Satisfaction Questionnaire (DTSQ)11-15 (items 1, 4, 5, 6, 7 and 8 of DTSQ). The global score (ATSQ-S) provides a range of 0 (minimum) to 36 (maximum). In the ATSQ-C version (used to overcome possible ceiling effects16), the global score has a range of -18 (highest loss in satisfaction) to 18 (maximum gain).
The PCAS (Appendix 3), adapted from the Perceived Competence Scale (PCS), is a short, 4-items questionnaire that assesses feelings of competence about self-implementing a treatment regimen.3,17 The score is calculated by averaging the responses on the 4 items.
Statistical analyses
The evaluable set consisted of all patients who fulfilled the selection criteria and received at least one dose of DA with SureClick®. The primary outcome was analysed in the subset of patients who had both the baseline and at least one post-baseline ATSQ-S score.
Descriptive analyses were provided for each outcome, together with their 95% confidence intervals (CI), at all the study visits.
Psychometric analyses of the ATSQ-S, ATSQ-C, and PCAS scales included: internal consistency (Cronbach’s alpha); factor analysis, using a varimax rotation (to verify that the adapted questionnaires have the same number of factors than the original questionnaires); test-retest reliability (analyzed between Record 1 and Record 2 in the subset of patients already self-administering the drug at all records); intraclass correlation coefficient; longitudinal validity (Pearson correlation between ATSQ-C at Record 2 and change in ATSQ-S between Records 2 and 0).
The mean change in ATSQ-S was compared between subgroups of patients with Student's Tests. Changes over time were assessed using Mc Nemar tests or paired t-tests. Statistical analyses were performed with the SAS® package-version 8.2. (SAS Institute, Cary, NC, USA).
Ethics
This study was conducted following the Declaration of Helsinki. Study protocol was approved by the Independent Review Board of the participating Institutions.
RESULTS
Population characteristics
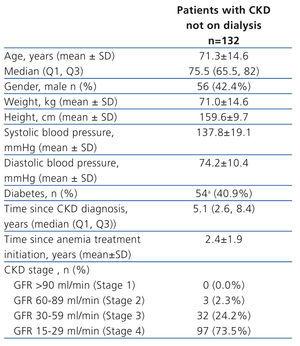
The study enrolled 132 evaluable patients (Table 1). Six patients were excluded from the analysis of the primary outcome (2 patients were lost to follow up, 3 patients died due to non-treatment related reasons, 1 patient discontinued due to adverse reaction). Median follow-up time (Q1;Q3) was 5.1 (4.1; 6.7) months. 57.6% of patients were receiving iron at baseline, and this percentage did not change during the follow-up. 86.2% of patients who were self-administering insulin at Record 0 (n=29) were also self-administering DA with prefilled syringes. Only three patients were self-administering DA but required assistance for insulin administration.
Satisfaction with treatment, perceived competence and self-administration degree
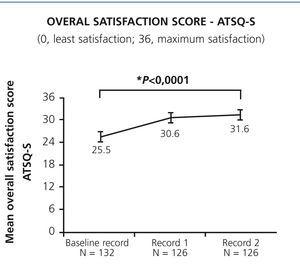
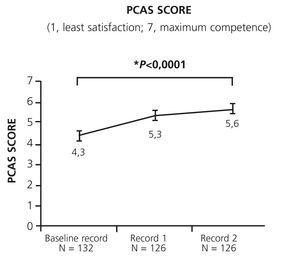
We observed a significant increase in patients’ perceived satisfaction and competence after switching to SureClick® (Figure 1 and Figure 2). The two items of the ATSQ-S with higher absolute changes were questions number 2 and 3 (mean change of +1.22 and +1.23 versus mean changes between +0.83 to +1.07 in the other items). The mean ATSQ-C score at Record 2 was 14.1 (5.3) points (95.1% (n=117) of patients with score >0). Mean (SD) PCAS scores also increased significantly (Figure 2).
Self-administration of DA was significantly increased (Table 2). The person responsible for administering DA with prefilled syringes in the 36 patients who achieved self-administration with SureClick® was: a health worker (at outpatient setting) in 44% (n=16) of cases, and a relative in the remaining 56% (n=20). Overall, at baseline record, 18% (n=24) of patients required the assistance of medical personnel (at home (1 patient) or at outpatient setting (23 patients)), and 34.1% (n=45) required a relative. These percentages were reduced to 5% requiring medical personnel (n=6, all at outpatient setting) and 20.8% (n=25) requiring help from a relative at Record 2.
Compliance with treatment and persistence with device
At Records 1 and 2, 96.2% and 98.4% of patients, respectively, had a compliance of 100%. 81.8% (n=108) of patients continued using SureClick® after the study (2 patients were lost to follow-up, 3 patients died, 1 patient discontinued due to adverse reaction and 18 patients did not specify the reason for not continuing with SureClick®).
Hemoglobin levels and doses of DA
No significant changes in Hb level were observed during the follow-up (baseline mean (SD) of 11.4 (0.5) versus 11.6 (1.3) g/dL at Record 2, p=0.193), although the dose of DA increased slightly (baseline mean of 17.1 (10.3) versus 18.4 (11.3) µg/week at Record 2, p<0.001).
Factors predicting satisfaction
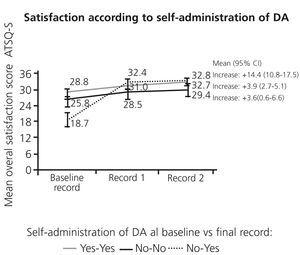
Patients who were not self-administering with syringes and achieved self-administration with SureClick® at Record 2 were those who had a greater increase in satisfaction (Figure 3) and perceived competence (data not shown). The increase in satisfaction and perceived competence was also associated with non-self-administration of insulin at baseline record, and with DA monthly administration frequency at Record 2 (data not shown). There were significant increases in satisfaction and competence in the subgroup of patients who ended with Hb≥10g/dL (mean change: 6.6, 95%CI: 5.0 to 8.3 for ATSQ-S and 1.5, 95%CI: 1.1 to 1.8 for PCAS, n=121), whereas no changes were observed in patients with Hb<10g/dL (mean change: 1.3, 95%CI: -3.8 to 6.4 for ATSQ-S and -0.3, 95%CI: -1.6 to 0.9 for PCAS, n=10).
No significant relationship was found between mean change in ATSQ-S or mean change in PCAS and the following variables: age, gender, compliance, iron administration (data not shown).
Psychometric properties of ATSQ-S, ATSQ-C and PCAS questionnaires
The three questionnaires had high internal consistency (Cronbach’s alpha coefficients at Record 2 of 0.90, 0.91 and 0.98 for ATSQ-S, ATSQ-C and PCAS, respectively). In the analysis of test-retest reliability, intraclass correlation coefficients were 0.62 (95%CI 0.34 to 0.78) (p<0.001) for the questionnaire ATSQ-S and 0.70 (95%CI 0.46 to 0.83) (p<0.001) for the PCAS. A significant correlation was found between the ATSQ-C at Record 2 and the change in ATSQ-S score between records 0 and 2 (Pearson correlation coefficient =0.47, p<0.0001). Patients who continued to use SureClick® after the study had a higher mean increase in ATSQ-S: 7.2 (8.4) versus 0.2 (9.3) points in patients who did not continue to use SureClick® (p=0.028, Wilcoxon test).
Safety
There were two adverse events probably related to treatment or device (1.5%), both due to injection site pain (one graded as mild and one graded as moderate). Only one of these patients discontinued prematurely due to the adverse reaction.
DISCUSSION
This is the first assessment of patient satisfaction and perceived competence with the use of SureClick® device in non-dialyzed CKD patients using three anemia-specific, multi-item questionnaires with good psychometric properties. The only previous single-center study assessing satisfaction with SureClick®,18 had already suggested a higher acceptance rate compared with prefilled syringes. Besides SureClick®, numerous autoinjection devices are currently available for other drugs.19-23 Previous studies with these devices indicate, in line with our findings, a higher level of patient satisfaction among users of prefilled pens versus traditional syringes,22,24,25 prefilled syringes,20,26 or even multi-use pens requiring cartridges.21,27,28
Both the competence and autonomy of patients for managing their anemia were significantly increased with prefilled pens. The PCAS showed a relative increase of 30.4%, which is clinically relevant since changes in this scale as small as 10% have been associated with improved clinical outcomes in trials of smoking cessation.17 The self-administration rate increased by 25 percentage points. These two measures of patient empowerment were interrelated. It is possible that the specific training performed by nurses or pharmacists at the baseline visit contributed to these positive results, but other studies with similar devices demonstrated that most patients achieve self-administration, even if they are not specifically trained within the setting of a clinical trial.20 The fact that the two items of the ATSQ-S with higher absolute changes were those asking about “convenience” and “flexibility” suggests that these two characteristics of the SureClick® device are those that most contribute to the increase in self-administration rate.
Since both the SureClick® and the prefilled syringes for administering DA are available at the same cost in all European countries, our results suggest that the SureClick® may be associated with significant savings of health costs due to a reduction in time of health professionals. At the end of the study, the need of assistance of a nurse to administer the injection had decreased by 13 percentage points, and the need of a relative was decreased in the same proportion. A previous French study with multi-use pens found that an increase in self-injection rates from 21% to 53% was associated, after a 2-months period, with productivity savings of 10.4 nurse hours.29
Our study indicates that the tolerability of the device was good. According to prescribing information, the overall incidence of injection site pain for DA is 7% in patients exposed for at least 6 months,30 and we found a rate of 1.5%, with 0.7% of discontinuation due to adverse reactions. This figure is lower than the 3.4% reported previously with multi-use pens for epoetin beta.5 The Hb levels did not change significantly over time, similar to previous findings in patients switching from prefilled syringes to SureClick®.31
Our study has some limitations. First, its observational, non-randomised design precludes drawing firm conclusions, however provides insight into the results that may be seen in the clinical practice setting. A cross-over design using ESA naïve patients could have reduced some of the biases inherent with a one cohort design but it would have required randomization within a clinical trial setting, and we preferred to obtain data as similar as possible to the real-life setting. Second, the fact that the patients were being observed as part of a study could influence their preference. The improved satisfaction with the SureClick® may have been due to its novelty, the enthusiasm of the investigators, and/or the improved training because it occurred during the study whereas the training with prefilled syringes (which is also performed routinely in the participating centres) may not have been as goal-oriented towards self-administration. Third, some possible selection bias in the patients could have occurred if the physician selected patients with better prognosis to use the SureClick® device, those more favourable to self-administration, or those less satisfied with prefilled syringes. These biases, if present, could have led to an overestimation of the preference and satisfaction with SureClick®. However, the fact that the baseline ATSQ-S score was quite high in our cohort suggests that enrolled patients were already quite satisfied with the use of prefilled syringes.
In conclusion, these results suggest that the use of SureClick® device for s.c. administration of DA in non-dialyzed patients with CKD could increase patient satisfaction and perceived competence for the management of anemia, without compromising either safety or treatment efficacy. These benefits could be associated with a high compliance rate. The change from prefilled syringes to SureClick® could also be associated with an increase in self-administration of drug, which could reduce the use of health resources, including family care as well as nursing and hospital resources. Future randomized controlled trials are needed to confirm our results.
Acknowledgements
This study was supported in part by a grant from Amgen, S.A. Writing assistance was supported by Neus Valveny from Trial Form Support.
Co-authors. This study has been conducted by the above-signed authors and the PREFERENCE study group (in alphabetical order of the principal investigator): Dr. Alberto Martínez Castelao, Carlos Ortega, Maria Sarrias (Hospital Universitari de Bellvitge); Dra. Cristina Vega (Hospital Galdakao); Dr. Enrique Andrés, Anna Maria Ramírez (Fundació Puigvert); Dr. Francisco Guerrero, Rosa María López (Hospital Torrecárdenas); Dra. Gregoria del Campo Cortijo (Hospital Carlos Haya); Dr. Javier Arrieta Lezama, Dr. Ramón Ortiz de Vigón, Dra. Olga González Peña, Dra. Inmaculada Ugarte Aróstegui, Dr. Iñigo Moina Eguren, Dr. Mikel Pinedo Olabarría, Eulalia Llaguno, Milagros Herrero, Concepción Arteche, Aurora Gurrutxaga (Hospital de Basurto); Dr. Joan Fort, Dra. María Rosa Gómez, Dra. María Asunción Galícia, Dra. María Eugenia Espinel (Hospital Vall d’Hebron); Dr. Josep M.ª Galcerán, Anna Maria Ramírez (Hospital Althaia); Dr. Juan Jesús Payán, Mariela Salas Robles (Hospital Costa del Sol); Dr. Manuel Manjón García, Pilar González Díaz, Dra. Gómez (Hospital San Cecilio); Dr. Manuel Ramírez de Arellano, Dra. Mònica Pou Potau, Marisa Lavado Sempere (Hospital de Terrassa); Dr. Marc Cuxart, Núria Boixader (Hospital de Figueres); Dra. Mercè Ardèvol (Hospital Germans Trias i Pujol); Dra. Silvia Collado,
Dr. Higini Cao, Dra. María José Soler (Hospital del Mar).
Conflict of interest
This study was sponsored by Amgen, S.A. J Claverol and M Cucala were employees of Amgen SA, Spain at the time the study was conducted. Both of them are former employees of Amgen SA, Spain.
Table 1. Main population characteristics
Table 2. Self-administration of DA at baseline record (using prefilled syringes) compared with final record (using SureClick®)
Figure 1. Change in patient&
Figure 2. Change in patient&
Figure 3. Change in satisfaction according to self-administration of DA at baseline and final records
Anemia Treatment Satisfaction Questionnaire (status version)(ATSQ-S) in English (1a) and Spanish versions (1b)
Anemia Treatment Satisfaction Questionnaire (change version)(ATSQ-C) in English (2a) and Spanish versions (2b)
Perceived Competence for Anemia Scale (PCAS) in English (3a) and Spanish versions (3b)
Appendix 1b. Spanish version
Spanish version
Spanish version