Antecedentes: Si bien la prevalencia de la enfermedad renal crónica (ERC) es del 10-14 %, diversos estudios prospectivos indican que en las fases 3 y 4 existe una tasa baja de progresión hacia enfermedad renal terminal (ERT). Una clasificación correcta del riesgo de progresión basada en factores predictivos demostrados permitiría un mejor manejo de la ERC. Estudios recientes han demostrado el elevado valor predictivo de la clasificación que combina el valor estimado (e) de la tasa de filtrado glomerular (FG) con la ratio albúmina-creatinina (RAC) en orina. Realizamos una estimación del riesgo clínico de una progresión hacia una ERT y de mortalidad cardiovascular existente en la población general española basando la predicción en el uso combinado de las variables tasa (e) de FG y RAC. Materiales y métodos: Evaluación cruzada en la muestra Epirce, que era representativa de la población española mayor de 20 años. Para la estimación del FG se emplearon las fórmulas MDRD y CKD-EPI; se consideraba la existencia de microalbuminuria cuando los valores de RAC oscilaban entre 20-200 mg/g (hombres) o entre 30-300 mg/g (mujeres) y de macroalbuminuria cuando los valores superaban dichos límites. Se realizó una estimación de la prevalencia ponderada poblacionalmente del riesgo de progresión de ERC hacia ERT. Resultados: Con MDRD, el 1,4 % de la población adulta española presentaba un riesgo moderado de evolución hacia ERT; el 0,1 % un riesgo elevado y el 12,3 % un riesgo bajo. Con CKD-EPI, la tasa de riesgo moderado se elevaba hasta 1,7 % y la de riesgo bajo hasta 12,6 %; sin embargo, la tasa de riesgo elevado se mantenía estable. Conclusiones: La adición de la RAC a la tasa (e) de FG permite una mejor clasificación de la población en riesgo de deterioro renal relacionado con el Kidney/Disease Outcomes Quality Initiative, grados 3 y 4. La estimación de la tasa de FG mediante CKD-EPI modifica la distribución existente para el riesgo bajo y moderado.
Background: Although the prevalence of chronic kidney disease (CKD) is 10–14%, several prospective studies note a low rate of progression to end-stage renal disease (ESRD) in stages 3 and 4. A correct classification of risk of progression, based on demonstrated predictive factors, would allow better management of CKD. Recent studies have demonstrated the high predictive value of a classification that combines estimated (e) glomerular filtration rate (GFR) and urine albumin–creatinine ratio (ACR). We estimated the clinical risk of progression to ESRD and cardiovascular mortality predicted by the combined variable of eGFR and ACR in the Spanish general population. Materials and Methods: This study was a cross-sectional evaluation in the Epirce sample, representative of Spanish population older than 20 years. GFR was estimated using MDRD and CKD-EPI formulas; microalbuminuria was considered to be an ACR 20–200 mg/g (men) or 30–300 mg/g (women) and macroalbuminuria was indicated beyond these limits. Population-weighted prevalence of risk of progression of CKD to ESRD was estimated. Results: With MDRD, 1.4% of the adult Spanish population was at moderate risk of progression to ESRD, 0.1% at high risk, and 12.3% at low risk. With CKD-EPI, the moderate risk ratio rose to 1.7% and low risk to 12.6%, but high risk remained stable. Conclusions: The addition of ACR to eGFR best classifies the population at risk for renal impairment relative to Kidney/Disease Outcomes Quality Initiative grades 3 and 4. Estimating GFR with CKD-EPI modifies the distribution of low and moderate risk.
INTRODUCTION
Chronic kidney disease (CKD) is a growing health problem in developed countries because of its high prevalence, effect on quality of life, and high vascular-related mortality,1 although its evaluation is imprecise. Classically, we assess the socioeconomic impact of CKD based on patients receiving renal replacement therapy (RRT),2 but the real burden of CKD is 50–70 times higher than those because of RRT,3,4 and patients in CKD stages 3–5 are at greater risk for cardiovascular disease (CVD) mortality than progression to end-stage renal disease (ESRD).5,6
The most frequently applied estimating formula, the Modification of Diet in Renal Disease (MDRD) equation,7 has been questioned because it underestimates GFR.8 The CKD-EPI creatinine equation is more accurate across various study populations and clinical conditions,9 and recent studies have shown an improved accuracy of CKD-EPI for estimating cardiovascular events and mortality risk.10-12 Nevertheless, these methods remain inaccurate because the GFR itself is a poor indicator of renal function given that it does not exactly correlate with the rate of uremic toxin.13
Guidelines proposed in 2003 by the Kidney Disease Outcomes Quality Initiative14 and adopted in 2005 by the Kidney Disease Improving Global Outcomes (KDIGO) defined CKD as the presence of a GFR <60mL/min/1.73m2 or kidney damage persistent for more than three months, regardless of cause.
In 2010, the Chronic Kidney Disease Prognosis Consortium15 reported the results of the meta-analysis of the association using estimated (e) GFR and ACR with mortality in the general population,16 and in 2011, the results of the association with progression to ESRD.17 In addition, this effect is present in people older and younger than 65 years, which again contradicts the belief that the prevalence of CKD increases with age;18 that belief is likely an artefact of the estimation formula, the retention of a renal functional reserve up to age 80 years,19 and the fact that age is the seventh leading factor in RRT.20 Furthermore, the association of eGFR-ACR with progression to ESRD is continuous and independent of other risk factors.17
CKD is otherwise a silent process in its early stages, linked to very early development of vascular lesions associated with microinflammation and monocyte activation, with evidence to suggest a turning point for risk at GFR 75.6–89mL/min.21,22 Strategies to identify patients at risk would allow appropriate management programs of primary or secondary prevention aimed at not only changing the progression of CKD but also at decreasing the risk of CVD mortality. In 2009, Hallan et al.23,24 proposed a new clinical risk classification system that combined all GFR levels with ACR measurement.
The aim of the current study was to estimate the clinical risk of progression to ESRD and cardiovascular mortality predicted by the combined variable of baseline eGFR and albuminuria in a Spanish general population. We also tested whether CKD-EPI eGFR modified the estimation of risk prediction compared to MDRD eGFR.
SUBJECTS AND METHODS
Study population
Our study population consisted of 2244 individuals who have been enrolled in cross-sectional EPIRCE study and have a urine albumin to creatinine ratio (ACR) estimation. In the Estudio Epidemiológico de la Insuficiencia Renal en España (EPIRCE) study, a random sample, stratified by age, sex, and location was drawn from the 2001 Spanish Census.25,26 The recruited sample was adjusted to provide valid estimates of age and sex according to the distribution of the Spanish population in 2001. All participants were Caucasians.
Data collection
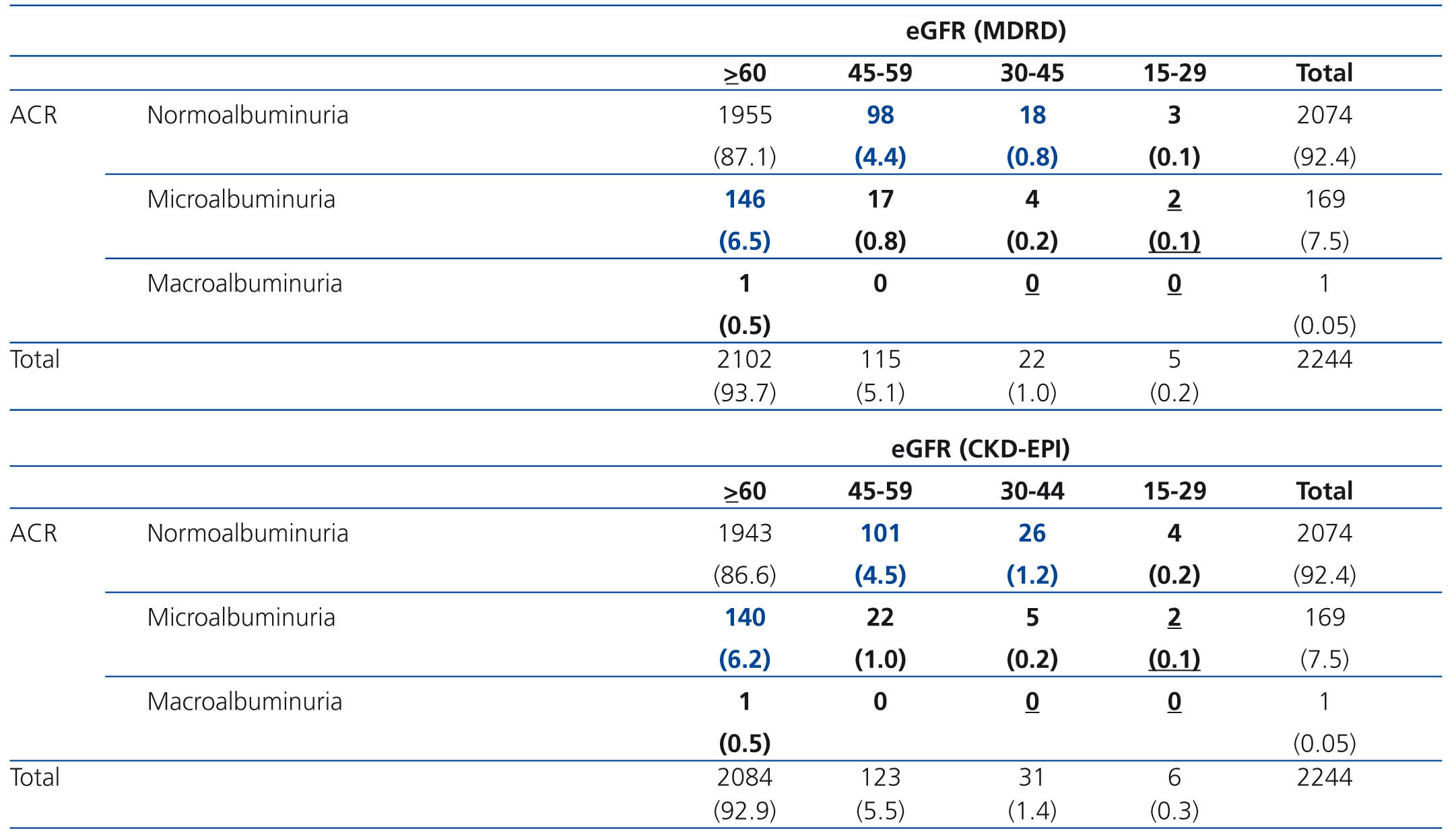
Data were collected using a standardized questionnaire administered during a structured interview, followed by a detailed physical examination and blood sample collection.25,26 Serum creatinine concentration was determined in the same reference laboratory for all samples. GFR was calculated as an indicator of renal function with the MDRD-4 formula, eGFRMDRD,7 and the CKD-EPI formula, eGFRCKD-EPI.9 Participants were classified (eGFR categories: ≥90, 60–89, 45–59, 30–44, 15–29, <15mL/min/1.73m2) according to the Kidney Disease Outcomes Quality Initiative guidelines.27 Patients were asked to deliver a spot urine sample, and ACR was used as an expression of albumin excretion. Microalbuminuria was defined as ACR 20 to 200mg/g in men and 30 to 300mg/g in women, and macroalbuminuria was defined as ACR >200mg/g in men and >300mg/g in women.28
A new CKD classification system with four categories of eGFR (≥60, 45–59, 30–44, and 15–30 mL/min/1.73m2) complemented by three categories of albuminuria (normoalbuminuria, microalbuminuria, and macroalbuminuria) was used for the description of low, moderate, and high risk of progression to kidney failure23 and the relative risk for cardiovascular mortality.24
Statistical analyses
Baseline subject characteristics are expressed as the mean ±SD or as percentages. Age- and sex-adjusted eGFR levels are reported as percentages or medians and percentiles.
We cross-tabulated eGFR using clinically relevant categories (≥90, 60–89, 45–59, 30–44, 15–29, <15mL/min/1.73m2) to evaluate the proportion of participants in each category of MDRD eGFR reclassified by the CKD-EPI equation eGFR. Generalized additive models (GAMs) (29) were used to evaluate the age and sex effect on eGFR categories, both eGFRMDRD and eGFRCKD-EPI.
The main advantage of GAMs over traditional regression methods is that they do not impose a parametric form on the effects of continuous covariates on the response of interest. Instead, they assume only that these effects are additive and reasonably smooth. In this paper, penalized regression splines combined with thin plate splines as smoothers were used to estimate GAM regression models, and the estimation of the smoothing parameters was chosen automatically by means of a generalized cross validation criterion. A Bayesian approach to uncertainty estimation was used to obtain 95% confidence intervals for the estimated effects.29 All statistical analyses were performed using R software, version 2.9.1; GAMs were fitted using the mgcv package.30
Ethical considerations
The Galician Ethical Committee for Clinical Research approved the study protocol. All participants provided informed consent.
RESULTS
The mean population age was 49.5 years, 52.6% (1445) were women, 25.8% (709) were 65 years old, and 16.6% (457) were 70 years old or older. Their clinical characteristics and lifestyles have been described previously (24), highlighting a regular consumption of alcohol (45.1%), smoking (25.5%), and physical inactivity (28.9%). The population has a high prevalence of dyslipidemia (29.3%), obesity (26.1%), and hypertension (24.1%), and 9.2% had a previous diagnosis of diabetes. Regarding previous cardiovascular events, peripheral vascular disease was the most frequent (10.8%), followed by ischemic heart disease (5.1%) and cerebrovascular disease (1.7%).
The prevalence of CKD stages 3 to 5 (eGFR <60mL/min/1.73m2) in the general Spanish population was 6.8% with eGFRMDRD and 6.9% with eGFRCKD-EPI; the prevalence of microalbuminuria/macroalbuminuria was 4.0%.
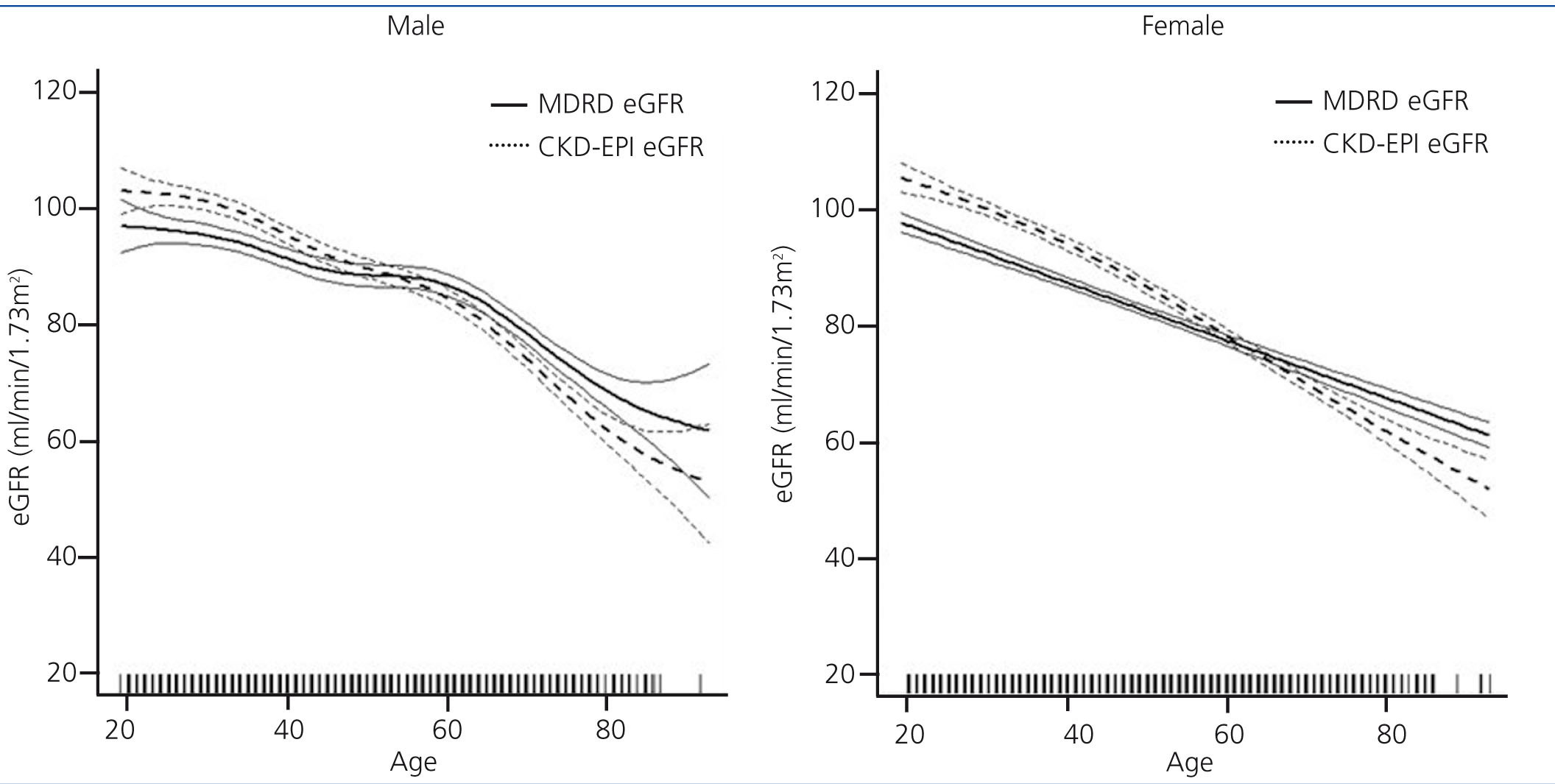
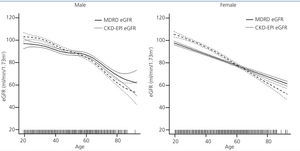
Population-weighted mean estimated GFR was higher when computed using the CKD-EPI equation (86.75mL/min/1.73m2; 95% confidence interval [CI] 86.06, 87.44) compared to using the MDRD formula (84.64mL/min/1.73m2; 95% CI 83.96, 85.31; p<0.0001). We also analysed groups by age and sex for eGFR variation between the two methods. The MDRD underestimated GFR values relative to CKD-EPI, but for people over age 60 years, eGFR results were similar and even slightly higher with MDRD (Figure 1).
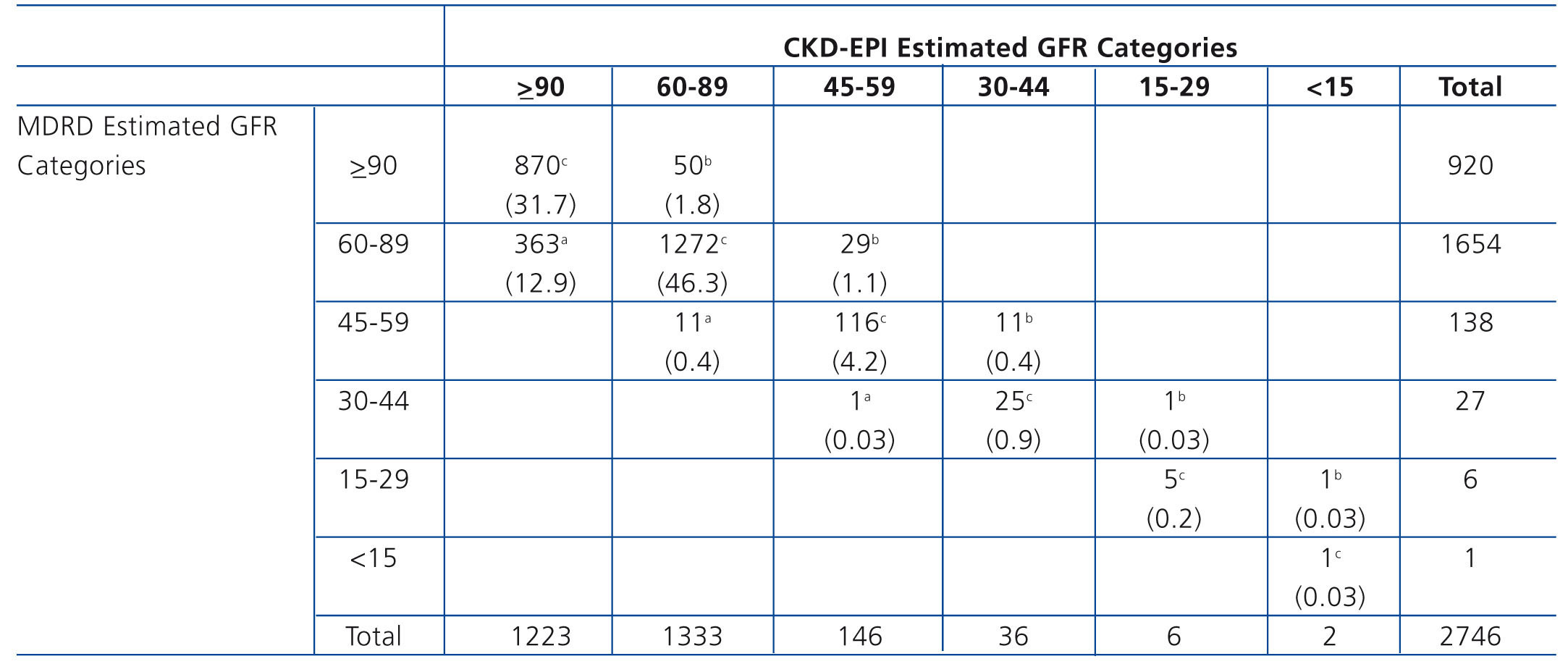
When we analysed the variation in eGFR categories as computed using CKD-EPI compared with MDRD, we found that 13.3% (365) of the population was reclassified to a higher eGFR category and 3.3% (92) was reclassified to a lower eGFR category (Table 1). Individuals reclassified to a lower category were older than those who were not reclassified (mean age 75.1±5.5 versus 49±17). When we analysed the subgroup of participants ages 65 years or older, we found that those reclassified to a lower category were older (75.5±5.1 vs. 72.6±5.2, p<0.001) and had more anaemia (6.1% vs. 1.6%, p=0.03), diabetes (41% vs. 23%, p=0.006) and a sedentary lifestyle (41% vs. 25%) compared to those who were not reclassified.
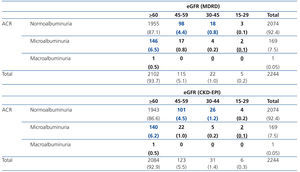
Table 2 shows the risk categories of progression to ESRD in the EPIRCE sample. The lower risk percentage was 11.7 with eGFRMDRD and 11.9 with eGFRCKD-EPI; the moderate risk percentages were 1.1 and 1.4, respectively; and the high risk percentage was 0.1 with both eGFR equations. We also assessed the age- and sex-weighted percentages of low (12.3% vs. 12.6%), moderate (1.3% vs. 1.7%), and high risk (0.1%) of progression to ESRD in the general Spanish population based on eGFR with the MDRD and CKD-EPI equations, respectively.
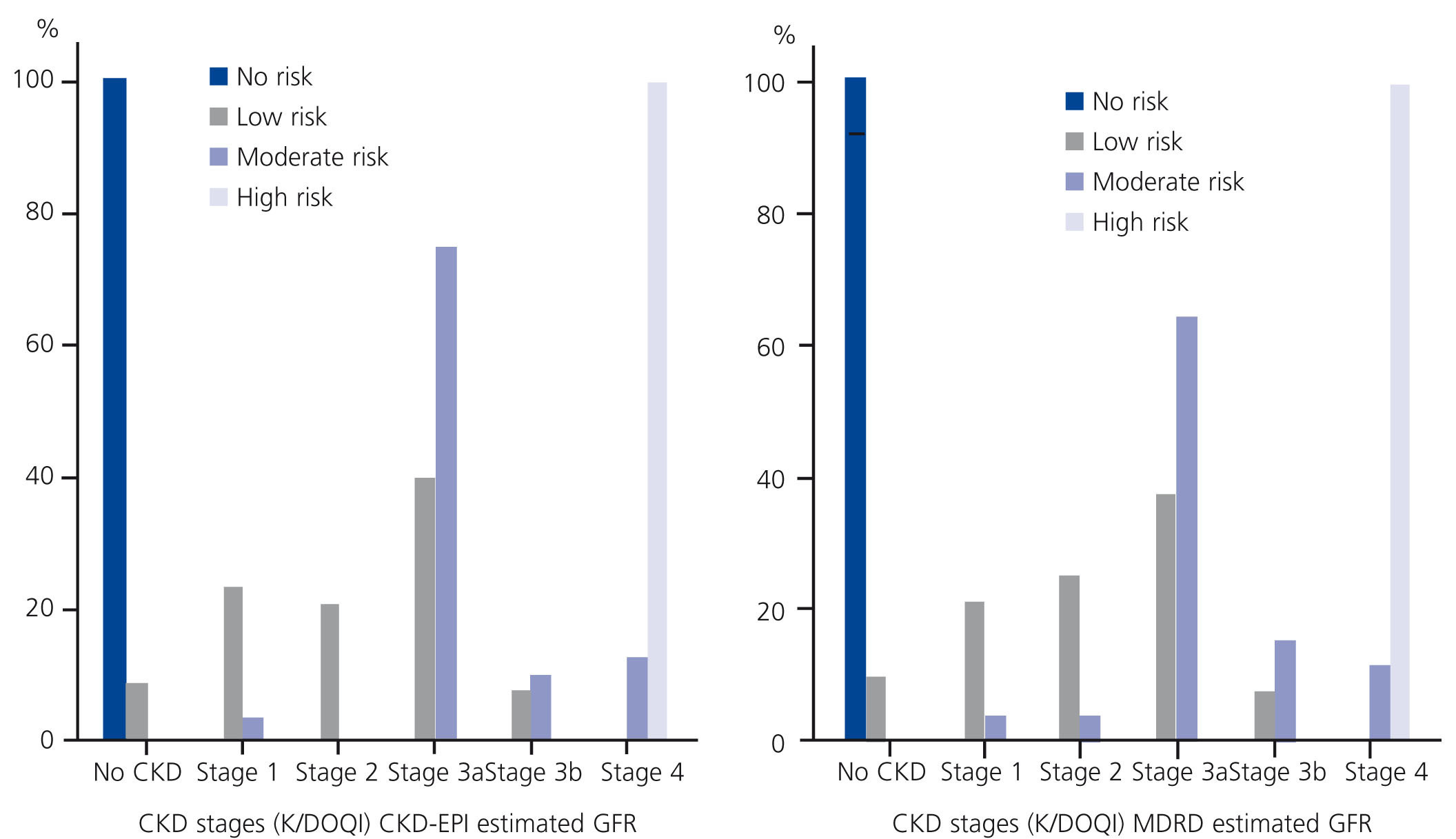
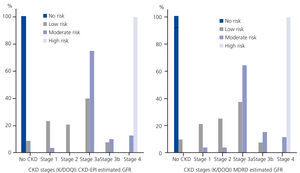
When we analysed the risk of progression to ESRD by CKD stages using eGFRCKD-EPI, we found that only 17.4% (15.0% with eGFRMDRD) of participants in CKD stage 3 presented a moderate risk of progression to ESRD, compared with 66.7% (60.0% with eGFRMDRD) in CKD stage 4 (Figure 2).
DISCUSSION
Using the stratification of risk of progression to ESRD given by Hallan et al.,23,24,31 12.6% of the Spanish population has a low risk of progression to ESRD while 1.7% has a moderate risk (with a hazard ratio [HR] of cardiovascular mortality between 2 and 3), and 0.1% a high risk (with a HR of cardiovascular mortality greater than 3). Although Hallan et al.’s proposal computed eGFR using the MDRD equation, recent studies have shown that CKD-EPI creatinine-based equation more accurately classifies individuals relative to risk for mortality and ESRD compared with MDRD, even after considering albuminuria.32,33 We computed the risk stratification of ESRD in the EPIRCE population by using both equations and the results show a slight increase in low and moderate risk percentages with eGFRCKD-EPI (12.6% vs. 12.3% and 1.7% vs. 1.3%) and the same percentage of high risk (0.1%) in the Spanish adult population. In previous studies, estimated GFR and ACR were the major predictors of future kidney failure, and adding age, sex, diabetes, hypertension, and other potential risk factors did not improve prediction.11,12,17 Risk stratification with a 12-category matrix (eGFR ≥60, 45–59, 30–44, and 15–29) subdivided by ACR into normoalbuminuria, microalbuminuria, and macroalbuminuria was more useful than the current CKD classification system.23,24,31,34,35 On the other hand, patients with CKD stage 1–3 have a 25 to 100 times higher risk of developing a cardiovascular event or death than of progressing to ESRD,5, 36 presumably because of subclinical atherosclerosis and/or vascular injury from early microinflammation.22
When CKD-EPI and MDRD estimation equations were compared in the current study, we found that MDRD underestimated GFR values relative to CKD-EPI, but over 60 years eGFR results were similar and even slightly higher with MDRD. The AUSDIAB study found that in Australians age >25 years, the reference population-weighted eGFR values were similar for the population age ≥65 years, regardless of which equation (MDRD or CKD-EPI) was used, but that the CKD-EPI equation yielded significantly higher reference values in younger age groups.37 Carter et al, in the East Kent population, observed mean eGFR using CKD-EPI equation to be 11.2% higher than that estimated using the MDRD Study equation for individuals aged 40-49 years; this difference gradually diminished to 0.7% in the 70-79 years old; and in people older than 80 years, the MDRD equation gave a lower CKD prevalence than the CKD-EPI equation.38 Kilbride et al. in European ancestry people older than 74 years found a mean lower eGFR using CKD-EPI equation than using MDRD equation (50.3 vs. 52.3mL/mn/1.73m2).39
We analysed the variation in eGFR categories when computed using CKD-EPI compared with MDRD. We found that 13.3% (365 participants) were reclassified to a higher eGFR category and 3.3% (92 participants) were reclassified to a lower eGFR category. These results are quite similar to those found by the Chronic Kidney Disease Prognosis Consortium's recent meta-analysis of 25 studies of population cohorts35 but with a higher percentage of people reclassified to a lower level (3.3% vs. 0.6%). Just as Matsushita et al. reported in that analysis,35 in our population the individuals reclassified to a lower level were older than those who were not reclassified (mean age 75.1 versus 49 years in EPIRCE population; 77 vs. 49 years in the meta-analysis population). When we analysed the subgroup ages 65 years or older, we found that those reclassified to a lower level were older and had more anaemia and diabetes and sedentary lifestyles than those not reclassified.
We acknowledge limitations to our approach as well. The cross-sectional nature of the study does not allow to us to draw conclusions regarding causality between lower GFR and cardiovascular or progression to RRT risk. But considering the sample representativeness, known in Spanish population behaviour of different estimation equations and the consequences of risk stratification as the proposal can be of interest to clinicians and health policy makers.
Different studies have shown36,40,41 that active disease management strategies can preserve kidney function and reduce CKD progression in CKD stage 3–5 patients Nevertheless, it remains unclear what the best clinically based criteria are that will result in more people benefiting from this approach. When considering the cost-effective use of health resources for the management of CKD, it is necessary to have instruments for selecting those patients in whom interventions are more efficient. A general practice and public health perspective favours the estimated GFR using the CKD-EPI equation.42 One strategy to be developed jointly by nephrology and primary care clinicians41,43,44 will be the establishment of consensus protocols to achieve this incorporation for the stratification of CKD patients. In the central area of Ourense, we have initiated a program for early detection of CKD and associated cardiovascular risk based on these criteria. Systematically, the primary care provider receives, together with eGFRCKD-EPI, an estimated risk of progression to ESRD according to risk stratification using ACR and eGFR. It will be necessary to assess the medium-term effectiveness of this program.
In conclusion, the proportion of the Spanish population with a high risk for progression to ESRD is low, but 1.7% is at moderate risk. The use of an instrument of "Risk Stratification of CKD Progression" employing the formula CKD-EPI+ACR would allow appropriate management not only in the early diagnosis of CKD but also as a tool for cardiovascular risk stratification and as criteria for referral from primary care to nephrology patients at risk of progression.
Acknowledgements
The coordinating investigators of the EPIRCE Study group in each Spanish Autonomous Community were as follows:
Andalucía, M.A. Álvarez-Lara; Asturias, F. Vega; Aragón, C. Laviades, P.J. Vives, J.M. Peña-Porta; Baleares, J. Marco, A. Solís, A. Losada-González; Cantabria, G. Fernández-Fresnedo; Canarias, J.F. Navarro, J.A. Sánchez-Joga; Catalunya, J. Fort, A. Martínez-Castelao, N. Fonseré; Castilla-La Mancha, F. Tornero, M. Quintana; Castilla-León, J. Grande-Villoria, A. Molina, B. Pozo, G. Torres; Ceuta y Melilla, C. Fernández-Andrade; Euskadi, F. Vidaur, J. Manrique, M. Rodríguez; Extremadura, F. Caravaca, B. Cancho; Galicia, A. Otero, L. González; La Rioja, A. Sánchez Casajús; Madrid, F. García, M. San-Boixedau, K. López, E. Rubio, C. Bernis; Navarra, J.L. Asín; and Valencia, J. Hernández-Jaras, A. Rius, M. González-Rico.
Conflict of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Distribution of estimated glomerular filtration rate (GFR) by age and sex. Differences by estimation equation in the EPIRCE Study.
Figure 2. Risk of progression to ESRD categories by CKD stages using eGFRCKD-EPI (left) and eGFRMDRD (right); the EPIRCE Study.
Table 2. Distribution of risk of progression to kidney failure in EPIRCE study population.
Table 1. Reclassification across eGFR categories using the CKD-EPI equation from categories based on the MDRD equation: the EPIRCE study