Thromboembolic phenomena are a serious complication of nephrotic syndrome (NS) with a general incidence of 20%.1 However, arterial thromboses (AT) are rare in adults.1,2 In this document, we describe multiple AT in an adult with NS. The genetic study pointed to an antithrombin Cambridge II (ACII) mutation. To our knowledge, this is the first case of NS with AT associated with this genetic thrombophilia.
The patient is a 73-year-old male who consulted for oedemas coursing for two to three weeks and re-exacerbation of dyspnoea in the previous few hours. Four weeks before he presented a right parietal lobe stroke (albuminaemia 2.7mg/dL). In terms of personal background, he was a smoker of 50 pack-years and had dyslipidaemia and arterial hypertension of several months’ evolution. His daily treatment included enalapril 20mg, atorvastatin 80mg, acetylsalicylic acid 100mg and furosemide 60mg. The following results were obtained in the physical exploration: blood pressure 120/73mmHg, oxygen saturation 97%, pitting oedema as far as the knee.
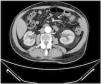
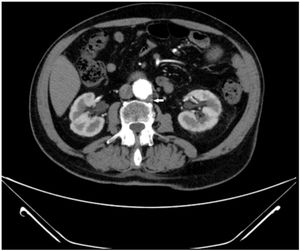
The analytical results were normal haemogram, fibrinogen 706mg/dL, D-dimer 1.7 microg/mL (nv [normal value]: 0.3–0.5), rest of coagulation normal; creatinine 1.1mg/dL, urea 39mg/dL, albumin 2g/dL, cholesterol 191mg/dL, triglycerides 80mg/dL, ions normal. IgG 389mg/dL, the other immunoglobulins were normal with no monoclonal component; complements, autoimmunity including anti-PLA2R, thyroid hormones, prostate-specific antigen (PSA), hepatitis markers, human immunodeficiency virus (HIV) and normal or negative lues serology. Proteinuria 6.59g/24h without a monoclonal component; sediment with one to three red blood cells per field. The electrocardiogram (ECG) and the echocardiogram were normal. The computed tomography angiography (CTA) of the pulmonary arteries/computed tomography (CT) of the chest showed a defect in the lateral segmental artery of the right basal pyramid consistent with pulmonary thromboembolism, whereby anticoagulation was initiated (suspended transiently as necessary); a nodular opacity of 10mm was also identified in the left upper lobe. The lower extremity (LE) Doppler ultrasound identified an aneurysm of the left femoral/popliteal arteries 2cm in diameter, partially thrombosed but no signs of deep vein thrombosis. The renal artery abdominal ultrasound/Doppler ultrasound showed no pathological findings in the renal echostructure, nor renal artery thrombosis. The thoracic-abdominal CT scan showed a 6-mm-thick mural thrombus in the infrarenal segment of the aorta (Fig. 1). Ten glomeruli were identified by renal biopsy; by optical microscopy and immunofluorescence lesions were compatible with membranous glomerulonephritis. The gastroscopy was normal; adenomas with low-grade dysplasia were identified in the colonoscopy and were resected. A polypectomy of several low-grade dysplasia adenomas was performed. In the thrombophilia study, the results for anticardiolipin antibodies (Ab), lupus anticoagulant, Factor V Leiden, protein C and S and the prothrombin gene were negative or normal, while antithrombin was 66% (NV 80%–100%), and the ACII (A384S) heterozygous mutation was identified by means of the allele-specific polymerase chain reaction assay followed by restriction analysis.
The pulmonary nodule was removed, and the histological study showed an in situ bronchogenic carcinoma. At discharge, anticoagulation with low molecular weight heparin was maintained; factor Xa levels showed adequate anticoagulation. After one year of follow-up, proteinuria of 6.5g in 24h persisted, with hypoalbuminaemia and preserved renal function, negative anti-PLA2R, with no tumour recurrence data, and rituximab 1g×2 was subsequently given.
Antithrombin is the body’s most important physiological anticoagulant factor with a molecular weight similar to that of albumin and both levels are correlated in NS.3–5 Antithrombin deficiency is present in 70% of cases of NS6 and promotes the multifactorial hypercoagulability status of this syndrome (Table 1) which usually induces venous thrombosis.1,7 On the other hand, in NS, AT is described in children and is usually in the lower extremities and coronary location; aortic involvement is very rare and potentially catastrophic.8,9 Some authors point to severe hypoalbuminaemia (1.6mg/dL) and the use of diuretics and corticosteroids as risk factors.2,5 Membranous nephropathy is not necessarily the most frequent cause of NS associated with AT.2,4 Our patient with popliteal vein, femoral artery and aortic thrombosis and ischaemic stroke had risk factors for AT such as dyslipidaemia, high blood pressure and smoking, and other thrombogenic diatheses involved in AT such as protein C and S deficiency or antiphospholipid syndrome were ruled out.8
Hypercoagulability of NS.
| Mechanisms: increase of factors: V, VII, von Willebrand, alpha-2 plasmin inhibitor, plasminogen activator inhibitor, fibrinogen |
| Urinary losses: antithrombin, possibly protein C and S |
| Increased platelet aggregation |
| Reduction in plasminogen |
| Nephropathies involved: membranous, membrano-proliferative, minimal changes, sclerosing and focal |
| Risk factors: membranousa, diuretics, steroids, repeated venepuncture, neoplasmsa |
| Venous location: renal vein (35%), pulmonary thromboembolism (8%), DVT LE (10%) |
| Arterial location: renal, aortic, femoral, cerebral, mesenteric, coronary |
| Prophylactic anticoagulation: in case of albuminuria <2g/dL |
NS: nephrotic syndrome; DVT LE: deep vein thrombosis of the lower extremities.
Moreover, ACII is due to a mutation in the antithrombin gene, which is of autosomal dominant inheritance and has variable penetrance. This mutation provokes a local functional and non-circulating deficiency of antithrombin and its prevalence is probably underestimated with routine diagnostic methods since it does not affect activity on the levels of the coagulation parameters.9 ACII is a moderate venous and arterial thrombotic risk factor9,10; in this latter territory, it appears to alter thrombin regulation capacity at the endothelial level. This dysfunction would be boosted in situations of hypercoagulability,9 such as NS and it could feasibly have played some kind of a role in this patient’s thromboses. From the therapeutic standpoint, in the presence of ACII, unfractionated heparin has apparently less anticoagulant efficacy than low molecular weight heparin.
The pathogenesis of arterial disease involves multiple genetic and environmental phenomena, which is why, in the presence of AT in NS, diagnostic possibilities permitting, the presence of pro-thrombotic genetic factors should be ruled out on account of their potential prognostic and therapeutic relevance.
FundingThe authors declare that they received no funding.
Conflict of interestThe authors declare that they have no conflict of interest.
Our thanks to Dr Jaime Corral, who performed the antithrombin genetic study, and to Dr Ricardo Enríquez for his collaboration.